INTRODUCTION
Chronic enteropathy (CE) is a common and challenging condition in veterinary medicine, characterized by gastrointestinal symptoms such as vomiting, diarrhea, weight loss, and altered appetite that persist for at least three weeks [1, 2]. Diagnosing and managing CE in dogs is often complicated by the overlapping clinical presentations and the limitations of traditional diagnostic methods [3, 4].
Conventional diagnostic tools for CE, such as blood tests, abdominal ultrasound, and endoscopy, provide valuable information but have limitations in visualizing the entire small intestine. Incomplete visualization can hinder accurate diagnosis and assessment of the disease’s extent [5].
Capsule endoscopy is a minimally invasive technique that involves the ingestion of a small, wireless video capsule, which captures real-time images as it traverses the gastrointestinal tract. This technology allows for the detailed examination of the small intestine, detecting lesions or abnormalities that may be missed by conventional methods [6, 7]. While capsule endoscopy has been widely utilized in human medicine for the diagnosis of obscure gastrointestinal bleeding and inflammatory bowel conditions, its application in veterinary medicine, particularly for the diagnosis of chronic CE, is still in its nascent stages [8, 9].
In this case report, we present a clinical approach using capsule endoscopy for the diagnosis and management of CE in two dogs.
CASE REPORT
A 10-year-old castrated male Maltese dog, weighing 3.8 kg, presented with chronic diarrhea, weight loss and abdominal distension at the Gyeongsang National University Animal Medical Center (GAMC).
The patient presented to a local animal hospital with a two-month history of chronic diarrhea, abdominal distension, and mild dyspnea. A clinical evaluation performed at a local animal hospital identified the presence of pleural effusion and ascites. Subsequent blood tests revealed significant hypoproteinemia and hypoalbuminemia. At the local animal hospital, repeated drainage of pleural effusion and ascites was performed, and corticosteroid therapy (prednisolone 1 mg/kg PO BID) was initiated; however, the patient showed minimal clinical improvement. Then the patient was referred to GAMC for a thorough diagnostic evaluation to identify the underlying cause and guide the treatment plan.
During the past two months, the patient exhibited significant weight loss, with body weight decreasing from 4.8 kg to 3.8 kg. On physical examination, the dog’s body temperature was within normal limits at 37.8°C. However, both respiratory and heart rates were mildly elevated. Auscultation revealed a grade 4/6 systolic murmur localized to the left apical region. Blood tests indicated elevated levels of symmetric dimethylarginine (43 µg/dL; reference range: 0–14 µg/dL), along with hypoproteinemia (4.7 g/dL; reference range: 5.2–8.2 g/dL) and hypoalbuminemia (1.8 g/dL; reference range: 2.2–3.9 g/dL).
Radiographic findings of the thorax and abdomen were consistence with suspeced pleral effusion and ascites. Abdominal ultrasound revealed hyperechoic changes with striations were identified in the mucosal layers of the jejunum, extending from the duodenum, with the most severe, focal involvement observed in the mucosal layers of the descending duodenum. Mild thickening of the gastric wall at the pyloric level was noted, measuring up to 9.3 mm, and the colonic wall showed thickening up to 2.5 mm. Additionally, anechoic free fluid was detected throughout the abdominal cavity. Pleural effusion in this patient was identified as a modified transudate, whereas ascites was characterized as a pure transudate.
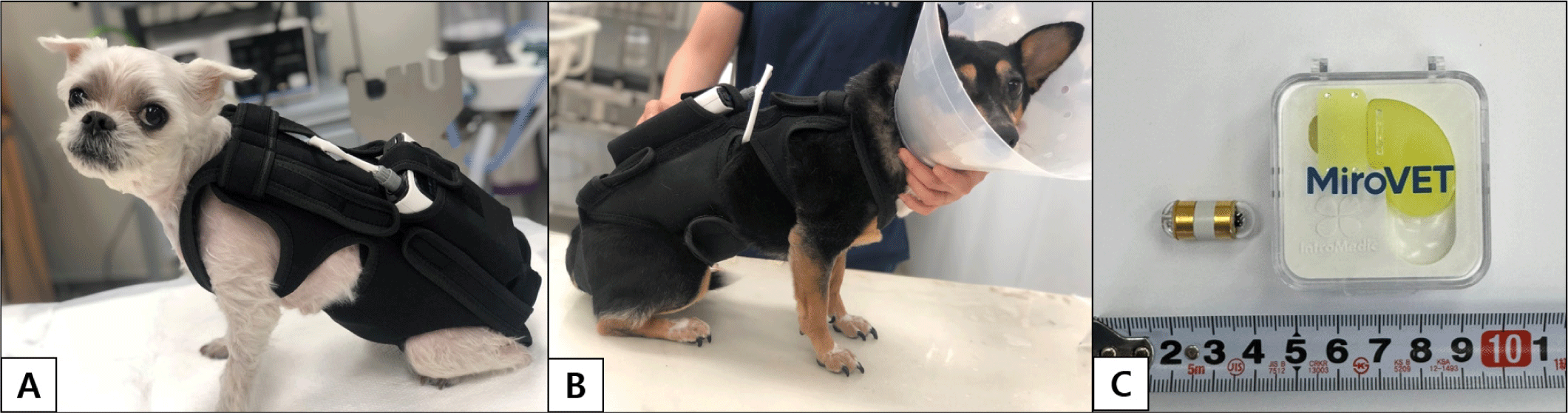
The patient’s Canine Chronic Enteropathy Clinical Activity Index (CCECAI) score was 14, indicating a severe disease status. Following a discussion with the client, it was decided to proceed with capsule endoscopy to further evaluate and differentiate underlying gastrointestinal disease. Then we proceeded with the capsule endoscopy examination (MiroVET®, VC1000, Intromedic, Seoul, Korea; length: 23.0 mm, diameter: 9.5 mm) to evaluate the overall mucosal condition of the intestine and for screening purposes (Fig. 1C). Prior to capsule endoscopic examination, the patient underwent a 12-hour fasting period, with access to water permitted. Simethicone (20 mg/dog) was administered 20 minutes before the procedure to reduce gastric gas and enhance image quality. The examination suit was then placed on the patient (Fig. 1A), and the capsule endoscope was administered orally.
During the capsule endoscopy, real-time monitoring was conducted via the viewer on the receiver. Once the procedure was completed, the data were transferred to a computer for detailed analysis. The total time for the capsule to reach the colon from the start of the procedure was 3 hours and 50 minutes.
The examination revealed mild erythematous changes in the gastric mucosa (Fig. 2A) and severe lacteal dilation throughout the small intestine, including the duodenum, jejunum, and ileum, with the most severe changes noted in the jejunum (Fig. 2B, C, and D). Due to the severity of the lacteal dilation, it was challenging to accurately assess changes in the small intestinal mucosa. However, when the mucosa was intermittently visible, it showed moderate to severe erythema and irregularity (Fig. 2E and F).
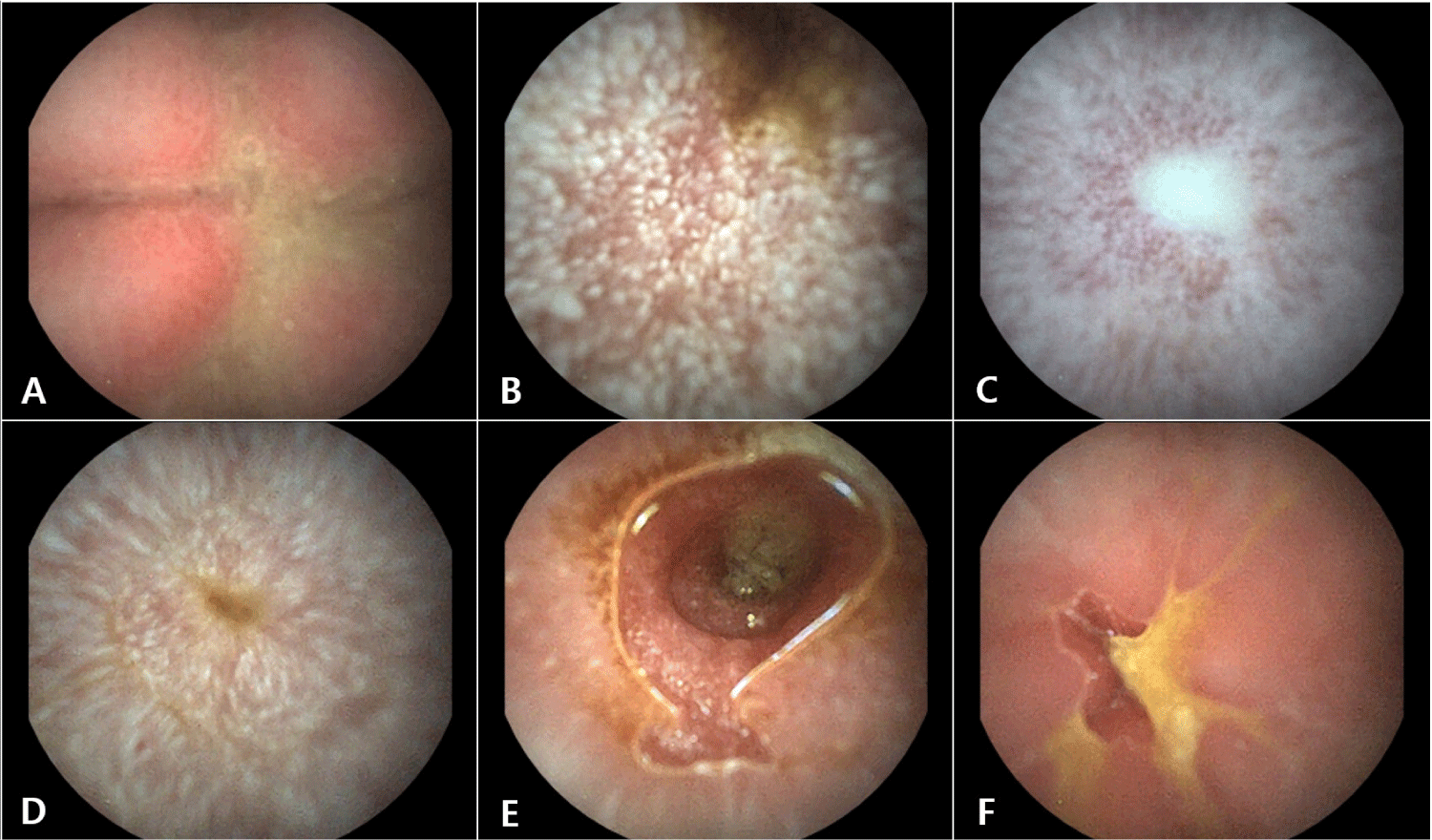
Based on the capsule endoscopy findings, the patient was tentatively diagnosed with mild gastritis, severe intestinal lymphangiectasia, and moderate enteritis.
As a result, we prescribed prednisolone (Solondo®, Yuhan Medica, Seoul, Korea; 1 mg/kg PO q 12 h), cyclosporine (Atopica®, Elanco, Greenfield, IN, USA; 6 mg/kg PO q 24 h) with diet change to low-fat diet (Low fat gastrointestinal, Royal Canin, Aimargues, France). After initial treatment, partial improvement was observed; however, the condition subsequently deteriorated and showed no response to medications.
To achieve an accurate diagnosis, we persuaded the client to consent to an upper gastrointestinal endoscopy under general anesthesia. The endoscopic findings were consistent with those observed in the capsule endoscopy (Fig. 3A). Mucosal biopsy samples were obtained from the duodenum using endoscopic forceps and submitted for histopathological evaluation (Fig. 3B).
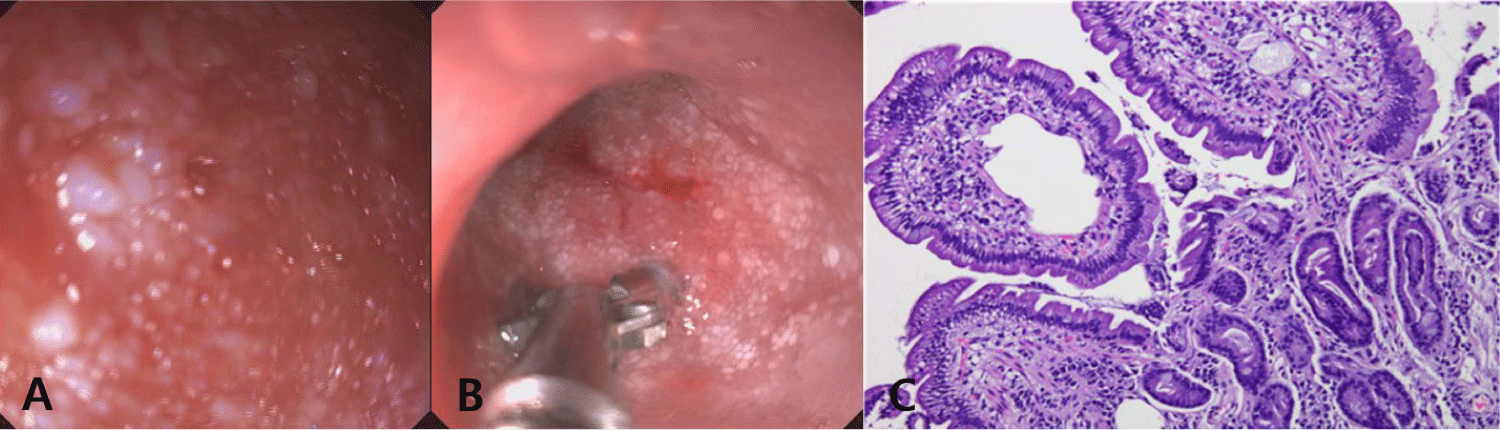
Histopathological analysis of the duodenal mucosa revealed moderate lacteal dilation and diffuse infiltration of inflammatory cells. High-power magnification demonstrated lymphocyte and plasma cell infiltration within the lamina propria, accompanied by mild fibrosis (Fig. 3C). No evidence of neoplastic cells suggestive of a tumor was observed.
We prescribed chlorambucil (3 mg/m2, once daily) for this patient, but only partial improvement in albumin levels was observed. Subsequently, despite increasing the dosage of chlorambucil (3 mg/m2, once daily), no further clinical improvement was noted. The patient continued to experience progressive accumulation of pleural effusion and ascites, receiving ongoing supportive care at a local clinic. The patient expired five months after diagnosis.
A 11-year-old neutered female mixed breed dog, weighing 5.6 kg, presented with chronic vomiting, anorexia, weight loss and intermittent abdominal pain at the GAMC.
This patient has experienced intermittent vomiting and loss of appetite over the past 3 to 4 years. Recently, however, the frequency and severity of vomiting and abdominal pain have increased, with a progressively worsening loss of appetite for three months. Over the last two months, the patient’s weight has declined from 6.1 kg to 5.6 kg. Despite the use of antiemetics and gastric acid suppressants at the local hospital, the symptoms showed no improvement. The patient was then referred to GAMC for a comprehensive diagnostic evaluation to determine the underlying cause and inform the treatment plan.
On physical examination, the dog’s body temperature was slightly higher than normal limits at 39.3°C. And, both respiratory and heart rates were mildly elevated. Auscultation revealed a grade 3/6 systolic murmur localized to the left apical region. Blood tests indicated elevated levels of symmetric dimethylarginine (21 µg/dL; reference range: 0–14 µg/dL), along with elevated blood urea nitrogen (48 mg/dL; reference range: 7–27 mg/dL). All other bloodwork parameters were within normal limits.
Thoracic and abdominal radiographs showed no abnormalities. Abdominal ultrasound demonstrated thickening of the entire small intestinal muscular layer, with corrugation observed in segments of the duodenum and jejunum. No other specific abnormalities were identified in the lymph nodes throughout the abdominal cavity. Multiple hyperechoic lesions with associated reverberation artifacts were noted, attributed to thickening of the gastric mucosal and muscular layers. The descending colonic wall exhibited localized thickening, measuring up to 3.9 mm.
The patient’s CCECAI score was 12, indicating severe disease. After consulting with the client, we decided to proceed with capsule endoscopy for a more comprehensive evaluation and to help differentiate the underlying gastrointestinal condition.
Then we proceeded with the capsule endoscopy examination (MiroVET®, VC1000, Intromedic; length: 23.0 mm, diameter: 9.5 mm) to evaluate the overall mucosal condition of the intestine and for screening purposes (Fig. 1C). Prior to the procedure, the patient underwent a 12-hour fasting period with access to water permitted. Simethicone (20 mg/dog) was administered 20 minutes before the examination to minimize gastric gas and improve image quality. The patient was then fitted with the examination suit (Fig. 1B), and the capsule endoscope was administered orally.
During the capsule endoscopy, real-time monitoring was conducted via the viewer on the receiver. Once the procedure was completed, the data were transferred to a computer for detailed analysis. The total time for the capsule to reach the colon from the start of the procedure was 2 hours and 30 minutes.
Capsule endoscopy revealed mild erythematous changes in the distal esophagus (Fig. 4A), along with diffuse erythema with erosive lesions of the gastric mucosa (Fig. 4B). In the small intestine, moderate to severe erythema and mucosal irregularity with edema were noted in the duodenum, jejunum and ileum (Fig. 4C). Persistent minor bleeding was noted in the erythematous areas of the duodenal and jejunal mucosa (Fig. 4D and E). In the colon, active and substantial bleeding was identified (Fig. 4F).
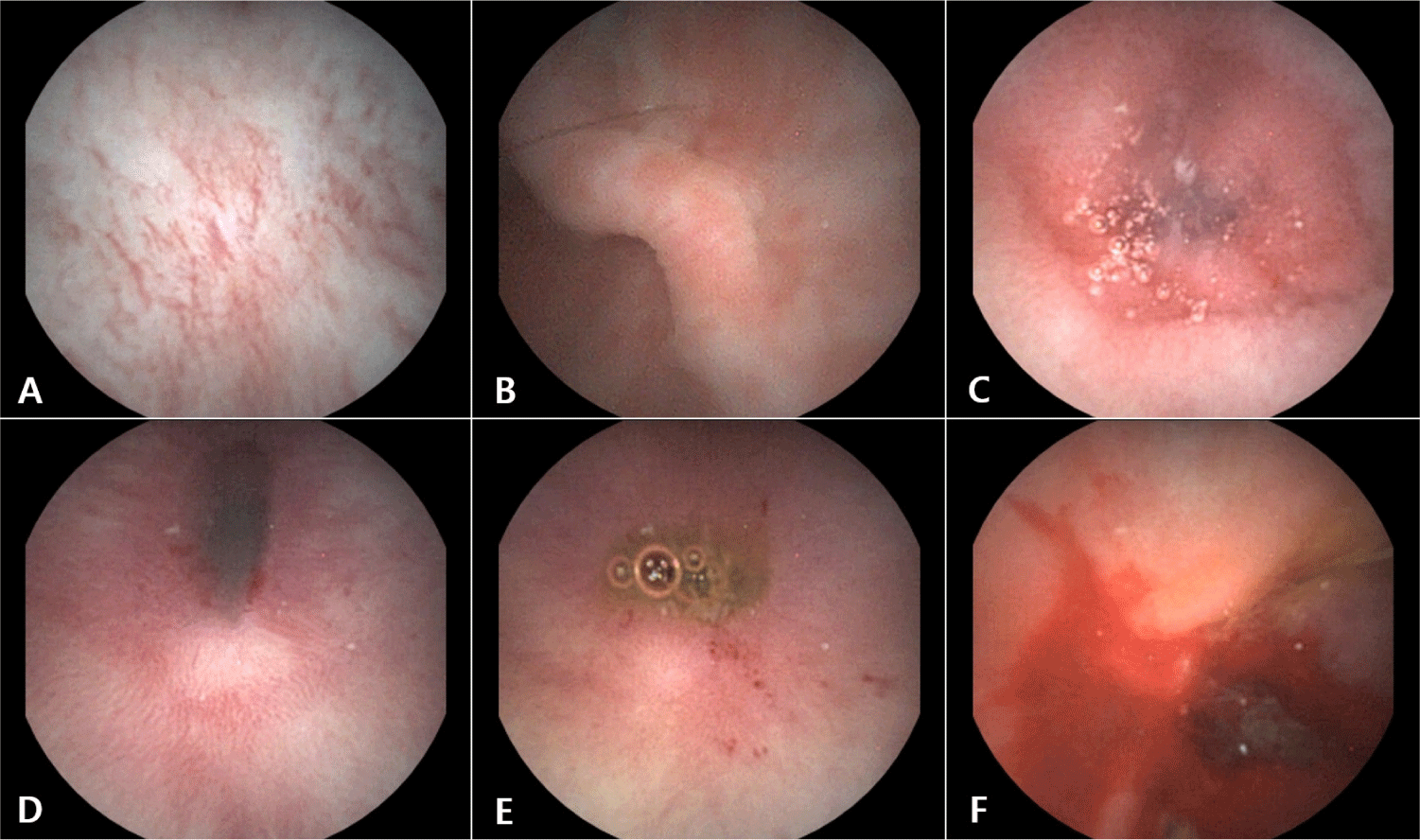
Based on the capsule endoscopy findings, the patient was tentatively diagnosed with mild esophagitis, moderate gastritis, and severe inflammatory bowel disease with evidence of intestinal hemorrhage.
After the diagnosis, a hypoallergenic diet (Hypoallergenic®, Royal Canin) was recommended, but due to palatability issues, it was substituted with Hill’s Z/D. However, soft stools persisted following the introduction of Hill’s Z/D. After discussing with the owner, the diet was changed to a low-fat option (Low fat gastrointestinal®, Royal Canin). We prescribed prednisolone (Solondo®, Yuhan Medica; 1 mg/kg PO q 12 h), omeprazole (Omed®, SK Chemical, Seongnam, Korea; 1 mg/kg PO q 24 h), and maropitant (Cerenia®, Zoetis, Parsippany, NJ, USA; 1mg/kg SC once then changed to PO for 5 days). The patient’s clinical symptoms gradually improved, and the steroid dosage was tapered accordingly.
DISCUSSION
In this case report, we demonstrated the application of capsule endoscopy as a non-invasive diagnostic tool in the evaluation of CE in dogs. Both cases highlighted the advantages of using capsule endoscopy for direct visualization of the small intestine, allowing for a more comprehensive assessment of mucosal abnormalities than traditional methods such as ultrasound. In case 1, the macroscopic findings of capsule endoscopy and gastrointestinal endoscopy were consistent, and the histopathological examination results also showed similar findings. The findings of severe lacteal dilation, extensive mucosal erythema, and irregular mucosal patterns provided crucial diagnostic insights that influenced subsequent treatment decisions.
Our results align with previous studies that have reported the effectiveness of capsule endoscopy in detecting mucosal lesions associated with gastrointestinal diseases in dogs [7]. The visualization of severe lacteal dilation, as seen in both cases, is a hallmark of advanced intestinal lymphangiectasia, a condition that often leads to protein-losing enteropathy (PLE). This finding is consistent with literature suggesting that lymphangiectasia is a common underlying pathology in severe chronic enteropathies [1, 2]. In a comparative review of canine intestinal lymphangiectasia, Jablonski [1] emphasized the diagnostic challenge of lymphangiectasia due to its diffuse nature, which often eludes detection through conventional imaging techniques [1]. In cases where lymphangiectasia is localized rather than diffuse, conventional gastrointestinal endoscopy can be challenging for diagnosis. In such instances, capsule endoscopy provides valuable diagnostic insights.
The integration of capsule endoscopy findings into the management of CE aligns with Dandrieux [10], who discussed the variability in treatment outcomes based on clinical response types. Food-responsive enteropathy (FRE) is the most common, with dietary trials often resulting in rapid and sustained improvement, whereas immunosuppressant-responsive enteropathy or non-responsive enteropathy (NRE) requires more complex interventions [10]. This distinction was evident in our cases, particularly in case 1, where dietary change and corticosteroid therapy was inadequate, necessitating additional therapeutic agents. Ultimately, the case was classified as NRE due to treatment failure, aligning with previous studies that emphasize the importance of tailored immunosuppressive therapies [10].
In both cases, accurate diagnostic testing was difficult due to the owners’ refusal to permit anesthesia; therefore, capsule endoscopy was conducted as an alternative screening test. In case 1, due to poor therapeutic response, the owner subsequently agreed to anesthetized upper gastrointestinal endoscopy and histopathological examination. These examinations revealed findings similar to those previously suggested by capsule endoscopy. In case 2, although biopsy and definitive diagnosis were not performed, capsule endoscopy provided valuable information that allowed assessment of the general mucosal condition. Therefore, while capsule endoscopy cannot accurately differentiate neoplastic lesions or provide definitive diagnosis, it appears to be useful as an alternative method for monitoring the overall intestinal mucosal condition and assisting in therapeutic decision-making.
As highlighted in the literature, diet modification remains a cornerstone in the management of CE. Dandrieux and Mansfield [11] emphasized that dietary interventions often yield the most favorable outcomes in FRE cases, particularly when hydrolyzed or novel antigen diets are employed [11]. This approach was supported by our cases, and although both patients received concurrent medical treatment, dietary modifications likely contributed to the stabilization of clinical signs [1, 11].
One of the notable advantages of capsule endoscopy in this context is its ability to visualize the entire length of the small intestine, including the deeper segments like the jejunum and ileum, which are often inaccessible with conventional endoscopy [5–8]. This extended visualization capability allows for the detection of subtle lesions, such as diffuse erythema or irregular mucosal patterns, that may be overlooked by other diagnostic methods. The findings from our cases support the growing body of evidence that capsule endoscopy can enhance diagnostic accuracy in chronic gastrointestinal conditions by providing high-resolution, real-time images of the intestinal mucosa [8, 9]. Moreover, capsule endoscopy allows for a thorough visual assessment of the entire gastrointestinal tract and facilitates the detection of ulcerative and hemorrhagic lesions, especially in patients for whom general anesthesia is risky or difficult to perform.
However, capsule endoscopy is not without limitations. One major drawback is the inability to obtain histopathological samples, which remain the gold standard for confirming specific diagnoses such as chronic inflammatory enteropathy or neoplastic conditions. In our cases, histopathological examination through upper gastrointestinal endoscopy was performed in the first case, whereas histopathological data were not obtained in the second case. Despite this limitation, capsule endoscopy provided critical visual information that guided clinical decision-making and helped in formulating targeted treatment plans [5–8].
The clinical utility of capsule endoscopy in monitoring therapeutic response has been well-documented in human gastroenterology, particularly in the context of a “treat-to-target” strategy, which aims for mucosal healing as an endpoint [8]. Similar approaches could be adapted in veterinary practice, especially for chronic cases where ongoing assessment of mucosal changes may help in tailoring treatment regimens and improving long-term outcomes. In our two cases, follow-up capsule endoscopy was not performed as part of the monitoring process. However, capsule endoscopy appears to have potential as a non-invasive tool for both initial diagnosis and follow-up monitoring in dogs with CE.
The CCECAI was utilized to assess disease severity and monitor treatment response in both cases. In case 1, the CCECAI score was 14, indicating severe disease, while in case 2, the score was 12. High CCECAI scores have been associated with poorer prognosis and higher risk of mortality, particularly in dogs with PLE [3, 4]. The ability of capsule endoscopy to detect extensive mucosal abnormalities was somewhat correlated with high CCECAI scores, suggesting its potential utility in assessing disease severity. Additionally, a major advantage of capsule endoscopy is that it can be performed non-invasively and without anesthesia, which is especially beneficial for patients in poor systemic condition.
In conclusion, this case report illustrates the diagnostic application of capsule endoscopy in the clinical management of CE in dogs. This report demonstrates that capsule endoscopy can be utilized as a non-invasive screening tool for evaluating the overall mucosal status of the intestines without anesthesia in patients with CE. Future studies with larger cohorts and longer follow-up periods are warranted to further evaluate the prognostic implications of capsule endoscopic findings and to refine its role in the management of chronic enteropathies in veterinary patients.