INTRODUCTION
Mesothelioma in situ (MIS) diagnosis is controversial, and characterized by the absence of a well-defined diagnostic criteria and limited understanding of its status as a true premalignant lesion in malignant mesothelioma progression [1]. Advances in the understanding of mesothelioma have raised awareness of MIS as a new entity that was not previously officially recognized, establishing a diagnostic criteria for MIS, which was included in the 2021 World Health Organization (WHO) classification [2]. Mesothelioma is a rare and highly aggressive neoplasm that arises from mesothelial cells, which line the serous membranes of the body. It is primarily associated with exposure to asbestos fibers [3–5] and has a long latency period, ranging from several decades to over 50 years [6]. This cancer can affect various serous membranes, including the pleura (lining of the lungs) [7], peritoneum (lining of the abdomen) [8], pericardium (lining of the heart) [9], and tunica vaginalis (lining of the testes) [10].
Shortness of breath is a common symptom of pleural MIS, and is often caused by malignant pleural effusion (MPE) [11, 12]. MPE is a build-up of fluid in the pleural space, which is the thin space between the lungs and tissue surrounding the lungs. As disease progresses, the tumor grows around the pleural surface, causing the tumor to surround the lungs, which leads to shortness of breath. Non-MPE can arise from effusion due to congestive heart failure or infection, independent of the primary tumor [13]. However, MPE in a clinical context generally occurs as a direct consequence of neoplastic conditions [14], such as primary malignant tumors in the pleural space (mesothelioma), or indirectly as a result of metastasis of other primary malignancies, including bronchogenic carcinoma infiltrating the lungs [13–15]. Additionally, hemorrhagic malignant pleural effusion (HMPE) is diagnosed in 47%–50% of all MPE cases [16].
Adenocarcinomas share certain characteristics with mesotheliomas as they both generate pleural fluid in the thoracic cavity. On cytology, mesothelial cells exhibit large cytoplasmic vacuoles that push the nucleus to the cell periphery, resembling the signet ring cells observed in some adenocarcinomas [17]. This similarity makes it challenging to differentiate between these two neoplasms. However, adenocarcinoma-induced pleural effusions are typically severe and associated with the metastatic (M1a) stage, which is characterized by radiographic evidence of the neoplastic lesion [18]. This distinction provides conclusive evidence to differentiate adenocarcinomas from MIS, in which no radiological evidence of a tumor is observed.
The remarkably broad morphological spectrum exhibited by reactive mesothelial cells sometimes overlaps with the morphological spectrum of malignant cells, leading to an increased probability of interpretive challenges and even false-positive results [17]. These challenges are further amplified by the presence of effusion-specific factors both in vivo and in vitro, which introduces additional complexities [17]. Notably, the term “malignant” is no longer used as a prefix for localized and diffuse mesothelioma [2]. However, according to the 2021 WHO classification of pleural and pericardial tumors, an MIS diagnosis of is based on the absence of pleural effusion (unresolved) and presence of imaging evidence indicating the presence of tumors within the thoracic cavity [2].
Mesothelioma has been extensively reported in humans, particularly because of its association with occupational exposure to asbestos [19]. However, reports on canine mesothelioma are rare. While the incidence of spontaneous mesothelioma in dogs is low [20], several cases have been documented [21–25], often presenting with unique clinical features and challenges in diagnosis and management. Treatment of canine mesothelioma is challenging, and no standard protocol has yet been established, with intracavitary platinum-based drugs reportedly being effective in some dog cases [26–28]. Intravenous injection and intracavitary cisplatin administration have also been trialed [26, 28]. However, the efficacy of these treatments is variable, and further research is required to determine the optimal treatment regimen.
This is the first reported case of MIS with HMPE in a dog in South Korea. Notably, this study displays the rapid accumulation of HMPE within the thoracic cavity, highlighting the urgent need for prompt diagnosis and intervention.
CASE REPORT
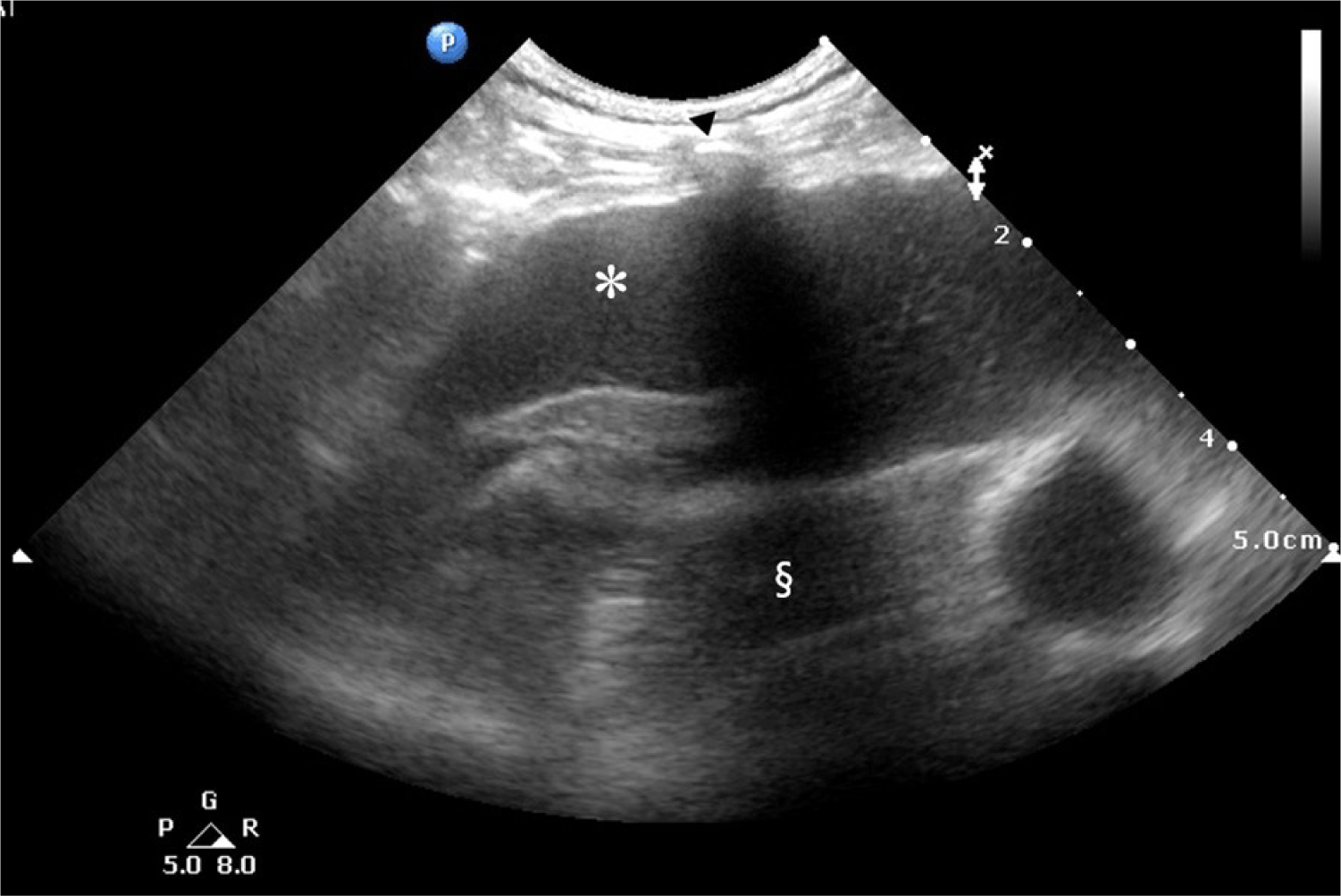
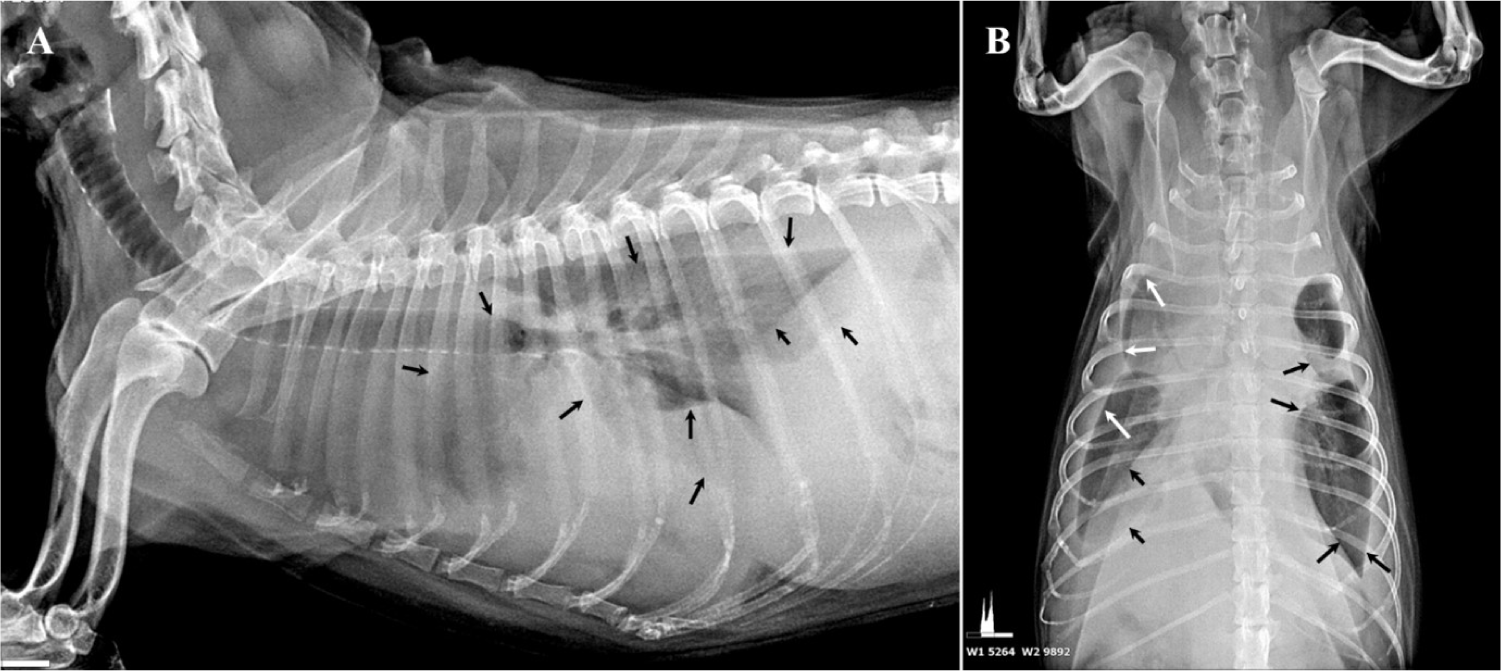
A 17-year-old spayed female Shih Tzu dog, weighing 5.0 kg, presented with a chief complaint of frequent coughing and respiratory distress. On physical examination, the patient was severely emaciated with a body condition score of 2/5, marked by prominent pelvic and rib bones. Considering the patient’s advanced age, initial auscultation was conducted to assess congestive heart failure as a potential cause of pulmonary edema. However, no abnormal heart sounds were detected, even though the patient exhibited deep, labored breathing, prompting further evaluation using thoracic ultrasonography. Ultrasonography revealed pleural effusion, rather than pulmonary edema (Fig. 1). Radiographic imaging revealed fissure lines in both left and right lung fields, consistent with pleural effusion. Notably, there were no interstitial patterns or radiopaque nodules (Fig. 2), which are characteristic radiographic features of neoplastic lesions in the thoracic cavity [29]. Comprehensive blood tests, including a complete blood count (CBC) and serum chemistry analysis, were unremarkable, except for thrombocytosis (platelets: 615 × 103/µL; reference range: 200–500 × 103/µL).
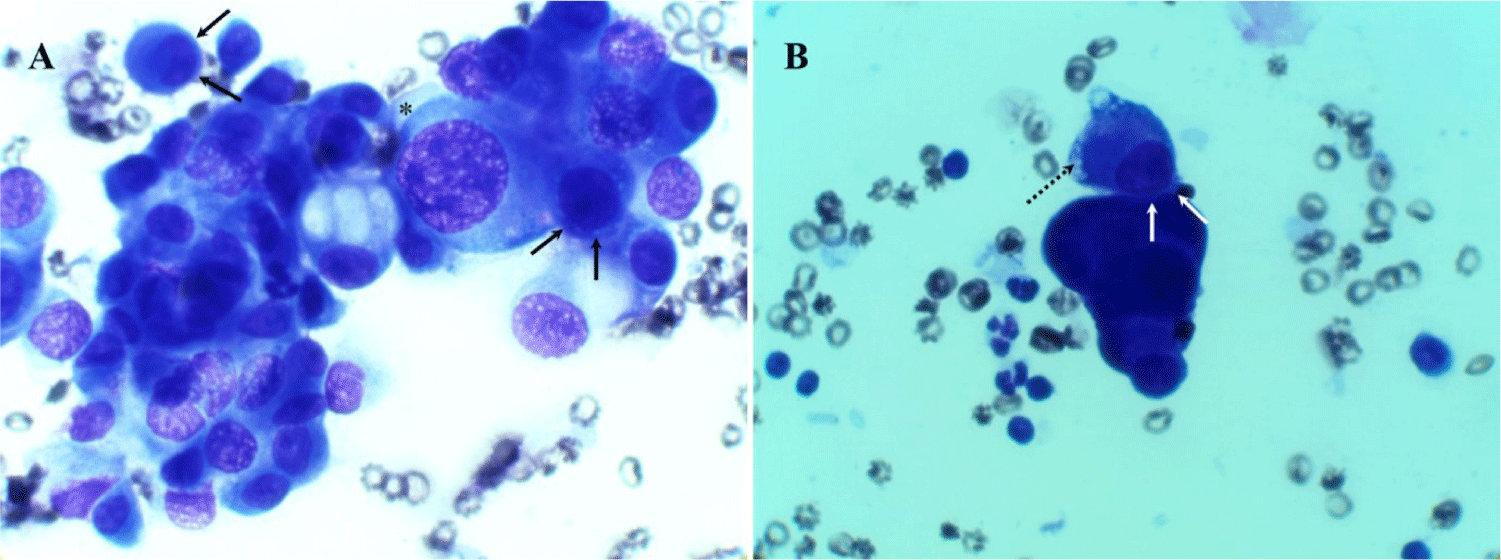
To alleviate the respiratory distress caused by pleural effusion, immediate thoracentesis was performed, removing > 200 mL of hemorrhagic serosanguinous pleural fluid (Fig. 3). Thoracentesis was performed between the 7th and 8th intercostal spaces to avoid the heart and liver, and it was conducted along the cranial edge of the rib to prevent damage to blood vessels and nerves. The puncture site was shaved, scrubbed, and prepared using sterile techniques. Pleural effusion was removed using the push-pull technique under ultrasound guidance, employing a blood access device a three-way Stopcock Valve (3-Way Stopcock Valve®, Hyupsung Medical, Seoul, Korea), a 10 mL syringe (BD Luer-LokTM Tip®, Becton, Dickinson and Company, Temse, Belgium), and a butterfly needle (Scalp vein set®, JMS Korea, Seoul, Korea). Post-thoracentesis, the patient’s breathing improved significantly. The pleural fluid, which was macroscopically classified as a modified transudate, was analyzed cytologically on smeared and stained glass slides. Cytological examination revealed marked anisokaryosis, a high nucleus-to-cytoplasm ratio, nuclear pleomorphism, and multiple nucleoli (Fig. 4A), suggestive of neoplastic MPE, potentially due to adenocarcinoma or mesothelioma. A notable two-zone cytoplasmic staining pattern with peripheral vacuolation was also observed (Fig. 4B). Additionally, the nuclei were eccentrically positioned and often in contact with the cell membrane, with a narrow cytoplasmic rim separating the nucleus from the cell border (Fig. 4A and B). Based on these cytological features and the absence of radiographic evidence of adenocarcinoma, MIS with HMPE was diagnosed.

Mesothelioma, a rare disease in dogs, is characterized by the development of malignant tumors originating from the mesothelial cells. Managing canine mesothelioma presents challenges owing to the lack of consensus on diagnostic protocols and limited information regarding therapeutic options [30]. In consultation with the owner, a treatment plan was discussed, which included the potential need for repeated thoracentesis to manage recurrent pleural effusion or if respiratory distress from effusion accumulation persisted, the possible placement of a chest tube.
To prevent secondary infections and manage inflammation, the patient received cephradine (25 mg/kg, SC; Cephradine inj®, Hanmi Pharm, Seoul, Korea) as an antibiotic and prednisolone (0.5 mg/kg, SC; Prednisolone inj®, Samu Median, Yesan, Korea) as an anti-inflammatory agent. Additionally, the patient’s owner was counselled to explore any potential association between mesothelioma diagnosis and asbestos exposure. However, no conclusive association was found between the patient’s living environment and asbestos.
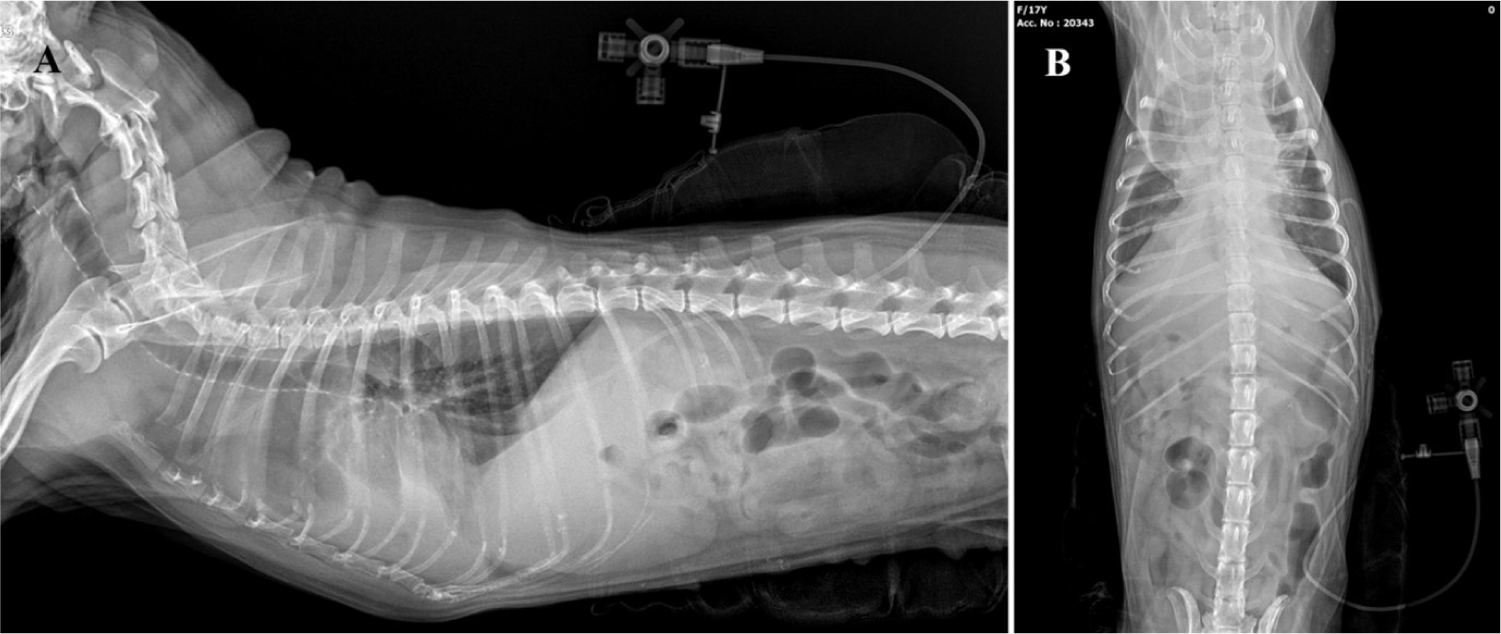
The patient was discharged with improved respiratory symptoms, and was scheduled for follow-up on the following day. A thorough examination was conducted during the follow-up. Despite initial improvement, the patient experienced recurrence of breathing difficulty after 1 d. Radiographic imaging confirmed recurrent pleural effusion, indicating that a single thoracentesis was insufficient as treatment. Consequently, a chest tube was placed to allow regular effusion drainage. A feeding tube (6 Fr; HMS Feeding Tube®, Korea Medical Supply, Seoul, Korea) was used as the chest tube. Local anesthesia was administered by infiltrating lidocaine (2% Lidocaine Inj®, Jeil Pharm, Seoul, Korea) at the insertion site along the left 5th–12th intercostal spaces. An incision was made between the 11th and 12th intercostal space, and right-angle forceps were used to guide the tube subcutaneously, positioning it through the 7th and 8th intercostal spaces (Fig. 5). The chest tube’s external connection was fitted with a three-way Stopcock Valve (3-Way Stopcock Valve®, Hyupsung Medical). Approximately 100 mL of hemorrhagic pleural effusion was drained daily through a chest tube.
To prevent infection, the patient was prescribed cephradine (25 mg/kg, PO, BID; Cefadin Cap®, Shin Poong Pharm, Seoul, Korea) as an antibiotic, and daily dressings were applied at the chest tube insertion site. Approximately 1 month after chest tube placement, the patient exhibited no signs of respiratory distress or related urgent symptoms. However, after 1 month, the owner reported that the chest tube had dislodged and that the hemorrhagic pleural effusion had drained from the insertion site. The patient also demonstrated breathing difficulties. Therefore, immediate transportation to a veterinary hospital was recommended; however, upon arrival, the patient had died. The option of a postmortem examination for histopathological confirmation was discussed with the owner, who declined and opted instead for cremation for funeral purposes.
DISCUSSION
This case involved a patient who presented with respiratory distress as the primary symptom. Considering the advanced age of 17 years, the initial diagnostic approach focused on eliminating congestive heart failure as a potential underlying cause. The absence of abnormal heart sounds on auscultation allowed the initial exclusion of cardiac disease. Subsequent imaging revealed significant pleural effusion, underscoring the need for further investigation through symptom management and effusion analysis. Thoracentesis yielded > 200 mL of effusion, which constituted > 4% of patient’s body weight. After drainage, the patient’s clinical symptoms improved significantly. The pleural fluid characteristics were consistent with a hemorrhagic modified transudate, supporting a diagnosis of HMPE [13, 16].
MIS is characterized by clinical signs such as weakness, pleural effusion, exercise intolerance, coughing, and anorexia in affected dogs. One study reported that a key feature of all canine mesothelioma cases was the presence of malignant transudates [23]. The average age of affected dogs is approximately 10 years, with no apparent breed or sex predilection. The clinical course of mesothelioma in these cases was relatively short, lasting approximately 1 month before euthanasia was performed [23]. Additionally, thrombocytosis is commonly observed on the CBC of dogs with mesothelioma [31], which is consistent with the findings in this case.
Differentiating MIS and primary respiratory adenocarcinoma as etiologies of MPE in the thoracic cavity is essential. Radiographic examinations are crucial to help distinguish MIS from adenocarcinoma. Morphologically, cytological differentiation between these two conditions can be challenging, as both may produce pleural fluid. However, MIS is typically characterized by dyspnea due to pleural fluid accumulation without radiographic lesions [2, 11, 12]. In contrast, adenocarcinoma-related pleural effusion generally appears at an advanced metastatic stage (M1a), when imaging would reveal tumor involvement [18]. Radiological diagnostic sensitivity and specificity for lung tumors have been reported to be 78.3% and 97.0%, respectively [32]. Considering the absence of radiographic evidence of pulmonary tumors in this case with serosanguineous pleural effusion, respiratory tract neoplasms were unlikely to be the cause of the effusion. Although the summation effect from the effusion influenced radiographic interpretation, it did not obscure potential indicators such as characteristic radiopaque masses or infiltrative patterns associated with adenocarcinoma.
Diagnostic imaging techniques such as computed tomography (CT) may aid in assessing thoracic effusion. However, although studies on thoracic CT imaging have shown that findings such as diaphragmatic thickening, nodular pleural thickening, and chest wall invasion are associated with malignant tumors in the thoracic cavity, it has limited diagnostic specificity for mesothelioma [33, 34]. In this case, CT was not performed because of the patient’s critical condition and severe dyspnea on admission, with immediate pleural fluid drainage taking priority. Once the patient’s condition stabilized, CT was considered; however, the owner declined further diagnostic procedures.
Although the cytological characteristics of mesothelioma-associated exudate are diverse, several key features are commonly observed [17]. These include marked anisokaryosis, high nuclear-to-cytoplasmic ratios, nuclear pleomorphism, and multiple nucleoli [17]. The nucleus is typically eccentric, often in contact with the cell membrane, with only a narrow rim of cytoplasm separating it from the cell boundary [17]. A two-zone staining pattern in the cytoplasm with peripheral vacuolation is also frequently observed, along with narrow cytoplasmic borders around the eccentric nucleus [17]. These cytological features are highly characteristic of mesothelioma-associated exudate and were consistently identified in the cytological analysis of the pleural fluid in the present case (Fig. 4).
Diagnosis of mesothelioma primarily depends on cytological examination, as distinct masses or tumorous effusions are rarely found in the affected cavities, such as the thoracic, abdominal, or pericardial spaces [35]. Additionally, obtaining biopsy samples for definitive diagnosis is often unfeasible or highly challenging [36, 37]. Thus, cytological examination is a viable and acceptable diagnostic approach for suspected mesothelioma.
In humans, mesothelioma most commonly originates in the pleura, and despite its aggressive nature, several chemotherapy regimens using cisplatin and pemetrexed have shown some improvement in survival rates [38–40]. In contrast, pleural mesothelioma has rarely been reported in dogs, partly due to a lack of consensus on diagnostic protocols and the disease’s rarity in this species [30]. Treatment options for canine mesothelioma are limited and generally focus on reducing effusion from a cytoreductive standpoint, with few documented therapeutic modalities [30, 41–44]. Chemotherapy was discussed with the patient’s owner; however, the absence of evidence-based literature confirming symptom improvement through chemotherapy limited its perceived benefits and ultimately precluded its administration.
The patient managed pleural effusion through daily drainage using a chest tube, either at home or in a veterinary hospital, with the pleural effusion being characterized as bloody serous and HMPE, consistent with the findings at initial thoracentesis [13, 16]. Approximately 100 mL of bloody pleural fluid (approximately 2% of patient’s body weight) was drained daily, allowing the patient to maintain respiratory stability and a relatively normal quality of life. However, a significant setback occurred when the thoracic tube was accidentally dislodged from the patient’s thoracic cavity. Despite daily dressing and use of an Elizabethan collar to prevent self-trauma, the patient died upon presentation after tube dislodgement.
Discussion with the owner yielded no conclusive explanation for tube’s removal, although it was speculated that the patient may have attempted to dislodge the tube because of discomfort when unobserved. Although general precautions for thoracic tube care were communicated to the owner, the potential for self-inflicted dislodgement was not anticipated. This case highlights a rare scenario, and in the absence of relevant literature or documented post-treatment guidance, the authors overlooked this aspect.
This case report is limited by the use of minimally invasive cytological diagnosis of pleural fluid for the diagnosis of mesothelioma, without confirmatory histopathological analysis or molecular testing. The absence of a postmortem histopathological examination with immunohistochemical staining poses challenges in differentiating reactive mesothelial cells, malignant mesothelioma, and adenocarcinoma. However, postmortem specimen collection was not feasible due to the owner's decision to withhold consent for autopsy.
As an alternative approach to address this limitation, a clinical methodology combining thoracentesis and radiological examinations was employed. Recent studies have highlighted the utility of minimally invasive techniques, such as the use of five immunocytochemical markers (cytokeratin, vimentin, desmin, E-cadherin, and calretinin), in the differential diagnosis of mesothelioma through pericardial fluid analysis obtained under ultrasound guidance [45]. Nonetheless, the limited availability of commercial laboratories offering immunocytochemical diagnostics, the time constraints for diagnosis (two days), and the financial burden (hundreds of dollars per marker) remain significant barriers to routine clinical application.
In this case report, an elderly patient presented with dyspnea as the primary symptom attributed to HMPE. To the best of our knowledge, this is the first reported case of MIS with HMPE in a dog in South Korea. Diagnosis was made through clinical and cytological evaluations, avoiding the need for anesthesia. Rapid accumulation of pleural fluid, a characteristic of MPE, poses a critical risk to the patient's life. To improve survival and stabilize the patient, a chest tube was inserted for active drainage of the HMPE, which proved to be effective in enhancing both survival and the patient’s overall quality of life.
Cytological analysis of HMPE considered both mesothelioma and adenocarcinoma as differential diagnoses. However, in the absence of radiographic evidence of thoracic tumors, combined with cytological features typical of mesothelioma, the patient was diagnosed with MIS with HMPE. The patient’s clinical symptoms improved significantly following chest tube insertion, which effectively alleviated pleural effusion pressure on the lungs. The patient maintained a satisfactory quality of life with the chest tube in place, exceeding the typical 1-month survival period for patients with MPE. Although the patient died, the management strategies and interventions used in this case offer valuable insights into the treatment of HMPE associated with MIS.