INTRODUCTION
Diethylbenzene (DEB, CAS No. 25340-17-4) is an almost colorless and flammable liquid with an aromatic odor. DEB is a byproduct of the synthesis of ethylbenzene from ethylene and benzene and contains a benzene ring and two ethyl substituents. It is primarily manufactured as a mixture of isomers (ortho: 1,2-; meta: 1,3-; and para: 1,4-) [1]. It is widely used in the production of synthetic rubber and resins as an intermediate or solvent in powderless etching [2], and is manufactured and/or imported in the European Economic area about 10 tonnes per year [3].
There are no data on DEB toxicity in humans, including skin or respiratory irritation, sensitization, or reported cases of occupational exposure. There is a high risk of exposure to workers through inhalation or skin absorption, with an estimated 60% of DEB is being absorbed through the respiratory tract, particularly in cases involving structurally similar chemicals [1]. There are few studies on the DEB mixtures because of the ratio difference between the three isomers; however, several studies on the toxic effects of each isomer in animals are available.
1,2-DEB is known to be the most toxic among isomers and causes central and peripheral neurotoxicity such as limb weakness associated with nerve fiber changes [4, 5]. Decreased leukocyte and lymphocyte counts were observed in rats exposed to the isomer mixture, and even though neurotoxic effect was not observed at the highest concentration (252 ppm), the researchers reported 10% of the highest concentration as no-observed-adverse-effect-concentration (NOAEC) of clinical neurotoxicity [1, 6]. Neither in vitro nor in vivo genotoxicity has been observed for the DEB isomer mixture [3]. Depending on the use of the substance to be manufactured, DEB is mainly exposed through the route of inhalation; however, there are limited data on repeated inhalation exposure, which was performed recently, compared to the oral exposure of animals. In addition, limited animal toxicity studies based on the OECD guidelines have been conducted. Therefore, we performed a 13-week repeated toxicity study to determine the toxic effects of DEB by inhalation exposure, according to the OECD Test Guideline No. 413, in accordance with Good Laboratory Practice (GLP) [7].
MATERIALS AND METHODS
Five-week-old specific-pathogen-free Wistar rats (40 males and 40 females) were obtained from SPF Biotechnology (Beijing, China) and acclimatized for two week in polysulfone cages (up to three animals of the same sex). The animal room was maintained at 22 ± 3°C, 50 ± 20% relative humidity, and a 12-hr light/dark cycle. Male and female rats weighed 235–275 g and 167–197 g, respectively, before exposure. The whole-body inhalation chambers (WITC-14M, HCT, Icheon, Korea) that were maintained at 22 ± 3°C and 50 ± 20% relative humidity with individual multi-compartment stainless steel wire mesh cages (W240 × L1200 × H200) were used for exposure. The chambers were vented 10–15 times per hour, and the oxygen concentration was maintained at least 19% during the exposure period. Animals were housed in polysulfone cages after the initial exposure and fed an animal diet (Teklad certified irradiated global 18% protein Rodent Diet 2918C, Envigo RMS, Indianapolis, IN, USA) and filtered water ad libitum. We also provided wooden chewing sticks to each rat as an environmental enrichment item. All animal care and inhalation studies were performed under GLP conditions, and the procedures were in accordance with the National Institutes Guide for the Care and Use of Laboratory Animals [8]. This study was approved by the Institutional Animal Care and Use Committee of the Inhalation Research Center, Occupational Safety and Health Research Institute, and all experiments were conducted according to the established institutional animal care and use protocol (Approval No. IACUC-2203).
DEB (mixture of isomers; 99.2% purity) was purchased from Alfa Aesar (Thermo Fisher Scientific, Waltham, MA, USA), and the ratio of isomer was unknown. The vaporized material was generated using a liquid-vapor generator (LVG-04-A, HCT), and the concentrations of the materials were analyzed using a gas chromatograph (Model No. TRACE1310, Thermo Fisher Scientific). The environmental conditions in the chambers were monitored every 30 min (Model No. ITC Manager, HCT).
The OECD guidelines (TG413) and the previously published method of Cho were used for the 13-week repeated inhalation study of DEB with modifications [9, 10]. A preliminary 28-day inhalation toxicity study was performed to determine the exposure concentration and showed no toxicological effect at a maximum concentration of 160 ppm, and a 90-day test was planned with reference to this results (unpublished). Male and female rats were randomly divided into four groups according to sex, with each group consisting of ten animals. Each group was exposed to 0, 40, 80, or 160 ppm DEB for 6 hr/day, 5 days/week, for 13 weeks. Concentrations of low (40 ppm), medium (80 ppm), and high (160 ppm) exposure groups were selected. All animals were observed once or twice daily (before and after exposure) to confirm detailed clinical signs, and body weight data were collected once or twice a week. Food consumption was measured once a week. At the end of the study, all the rats were anesthetized with isoflurane (I-Fran liquid, Hana Pharm, Hwaseong, Korea) and euthanized via exsanguination of the abdominal aorta and vein.
Blood samples were collected from the abdominal aorta before euthanasia. The samples were collected in tubes containing an anticoagulant (ethylenediaminetetraacetic acid or sodium citrate) for hematological parameters, or in serum-separating tubes for biochemical examination. The anti-coagulant tubes containing sodium citrate and serum separating tubes were centrifuged at 3,000 × g at 4°C for 10 min to separate the serum. Hematological or biochemical parameters were examined using blood cell analyzer (ADIVA 2120i, Siemens Diagnostics, Tarrytown, NY, USA), coagulation analyzer (ACL Elite Systems, Instrumentation Laboratory, Bedford, MA, USA), or blood chemistry analyzer (TBA-120FR, Toshiba, Tokyo, Japan): white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (HGB) concentration, hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet (PLT) count, reticulocyte (RET) count, differential WBC count (neutrophils, lymphocytes, monocytes, eosinophils, and basophils), activated partial thromboplastin time (APTT), prothrombin time (PT), sodium (Na), potassium (K), chloride (Cl), total protein (TP), albumin (ALB), creatinine (CREA), blood urea nitrogen (BUN), glucose (GLU), calcium (Ca), inorganic phosphorus (IP), total bilirubin (TBIL), total cholesterol (TCHO), triglyceride (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and albumin/globulin (A/G) ratio.
Bronchoalveolar lavage fluid (BALF) samples were collected immediately after euthanasia. The samples were collected from the right-sided lung by lavage with phosphate buffered saline and centrifuged at 3,000 × g at 4°C for 10 min to separate the cells and supernatant. Biochemical parameters including TP, ALB and lactate dehydrogenase (LDH), were examined using a blood chemistry analyzer, and total cells were counted using an automatic cell analyzer (NC-200, ChemoMetec, Allerod, Denmark). At least 400 cells, including alveolar macrophages, monocytes, lymphocytes, neutrophils, and eosinophils, were counted under a light microscope (Axio Scope, Carl Zeiss, Oberkochen, Germany).
During necropsy, the adrenal glands, brain, epididymis, heart, kidneys, liver, lungs, ovaries, spleen, testes, thymus, and uterus were examined and weighed. And the following tissues were also examined and collected in 10% neutral buffered formalin or Davison’s fixative (for the testes and eyes/optic nerves) for the histopathologic examination: aorta, bone marrow, cecum, colon, duodenum, esophagus, eyes/optic nerves, femur, gallbladder, harderian glands, ileum, jejunum, larynx, lung (left lobe only), lymph nodes (tracheobronchial and mesenteric), mammary gland, nasopharyngeal tissue, pancreas, parathyroids, pituitary, prostate, rectum, salivary glands (submandibular, sublingual, and parotid), sciatic nerve, seminal vesicle, skeletal muscle, skin, spinal cord, sternum, stifle joint, stomach, teeth, thyroid, tongue, urinary bladder, and vagina. According to the OECD TG413, all of the fixed tissues from control and high exposure groups of male and female rats were paraffin-embedded, 4 μm thickness-sectioned, stained with hematoxylin and eosin (Automatic Stainer, DAKO, Glostrup, Denmark), and examined under a light microscope (DM3000, Leica, Wetzlar, Germany). However, histopathologic examinations from low and medium concentrations were not performed in consideration of the effect of the test substance.
Data are expressed as mean ± S.D. Analysis of parameters, including body weight, food consumption, organ weights, and hematological or blood biochemical data was performed using the Pristima® system (Version 7.1.0, Xybion, Princeton, NJ, USA). Data were checked for homogeneity using Levene’s test and analyzed using with one-way analysis of variance (Dunnett’s multiple test) or Kruskal–Wallis (Dunn rank sum test). Differences were considered significant at p<0.05 or p<0.01.
RESULTS
The analytical concentrations of DEB in chambers during the exposure period for the low, medium, and high exposure groups were 39.48 ± 1.13 ppm, 80.43 ± 2.06 ppm, and 160.20 ± 4.42 ppm, respectively.
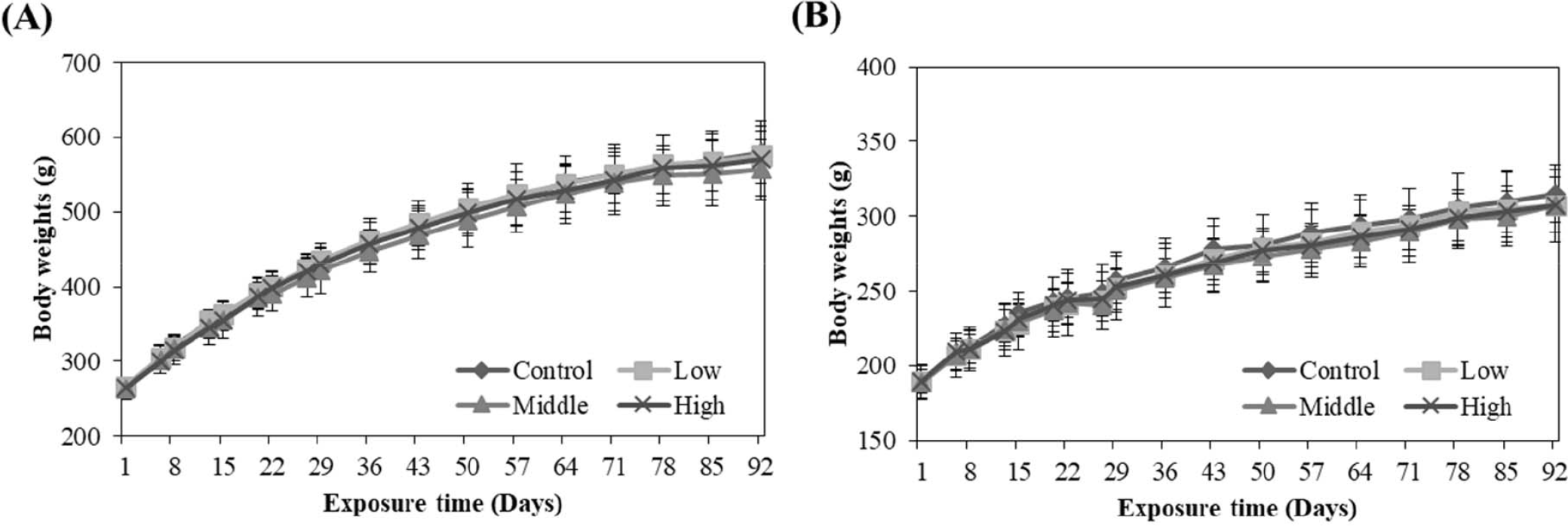
No animals died or unusual behavioral or clinical signs were observed during the study period (data not shown). In addition, there were no significant changes in the body weight (Fig. 1) or food consumption (data not shown) of male and female rats during the study.
The PT level was significantly increased in the medium- and high-exposure groups in male rats compared to that in control males (medium: p<0.01; high: p<0.05). Additionally, ALP levels in the male high-exposure group were significantly high (p<0.05). No significant differences were observed in other hematological or biochemical parameters between the control and experimental groups (Tables 1, 2, 3, and 4). In addition, there were no significant differences in BALF data between the control and experimental groups (Table 5).
n, number of animals examined; WBC, white blood cell count; RBC, red blood cell count; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelets; NEU%, neutrophil relative; LYM%, lymphocyte relative; MON%, monocyte relative; EOS%, eosinophil relative; BAS%, basophil relative; NEUA, neutrophil absolute; LYMA, lymphocyte absolute; MONA, monocyte absolute; EOSA, eosinophil absolute; BASA, basophil absolute; RET%, reticulocyte relative; RETA, reticulocyte absolute; APTT, activated partial thromboplastin time; PT, prothrombin time.
n, number of animals examined; WBC, white blood cell count; RBC, red blood cell count; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet; NEU%, neutrophil relative; LYM%, lymphocyte relative; MON%, monocyte relative; EOS%, eosinophil relative; BAS%, basophil relative; NEUA, neutrophil absolute; LYMA, lymphocyte absolute; MONA, monocyte absolute; EOSA, eosinophil absolute; BASA, basophil absolute; RET%, reticulocyte relative; RETA, reticulocyte absolute; APTT, activated partial thromboplastin time; PT, prothrombin time.
n, number of animals examined; Na, sodium; K, potassium; Cl, chloride; TP, total protein; ALB, albumin; CREA, creatinine; BUN, blood urea nitrogen; GLU, glucose; Ca, calcium; IP, inorganic phosphorus; TBIL, total bilirubin; TCHO, total cholesterol; TG, triglycerides; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; A/G ratio, albumin/globulin ratio.
n, number of animals examined; Na, odium; K, potassium; Cl, chloride; TP, total protein; ALB, albumin; CREA, creatinine; BUN, blood urea nitrogen; GLU, glucose; Ca, calcium; IP, inorganic phosphorus; TBIL, total bilirubin; TCHO, total cholesterol; TG, triglycerides; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; A/G ratio, albumin/globulin ratio.
n, number of animals examined; TC, total cells; MAC%, alveolar macrophage relative; LYM%, lymphocyte relative; NEU%, neutrophil relative; EOS%, eosinophil relative; MACA, alveolar macrophage absolute; LYMA, lymphocyte absolute; NEUA, neutrophil absolute; EOSA, eosinophil absolute; LDH, lactate dehydrogenase; TP, total protein; ALB, albumin.
The absolute organ weights or organ weights relative to the whole-body weight were investigated (Tables 6 and 7). In terms of absolute organ weights, no significant change was found between the experimental groups and the control group. The kidney weight in the low group was significantly lower than that in the control in regard to the relative organ weights. Gross findings were observed in the lungs, spleen, testes, and vagina (Table 8), and were identified by microscopic examination as alveolar macrophage aggregation in the lung, unclassified sarcoma in the spleen, seminiferous tubular dilatation in the testes, and cysts (s) in the vagina. Microscopic findings were also observed in the heart, liver, lungs, nasal cavity, pancreas, pituitary, testes, and thyroid vagina in the control and high exposure group of rats (Table 9).
DISCUSSION
DEB is a by-product of the synthesis of ethylbenzene from ethylene and benzene. It occurs as a mixture of three isomers and the ratio of each isomer varies according to the production process [11]. In the 1950s, the ratio of 1,2-DEB in the mixture was known to be about 25%, and studies in the 1990s showed that 6%–10% of 1,2-DEB was included in the mixture [1, 6, 12, 13].
In the animal toxicity studies, the LC50 of DEB mixture (25% 1,2-DEB, 40% 1,3-DEB, and 35% 1,4-DEB) was reported to be 2,100 ppm or higher after 7-hr single inhalation exposure. Several clinical signs, such as nasal irritation and dizziness were observed, and decreased body weight and blue-colored organs were noted [3]. In another study, the exposure of male and female SD rats to DEB by inhalation at 0, 34, 110, and 252 ppm resulted in decreased leukocyte levels in both sexes above 110 ppm. At a concentration of 252 ppm, bluish-colored organs and reduced body weight were identified, and there was a decrease in biochemical parameters, including ALT, AST, and CREA kinase activity in females. There were no adverse histological findings or neurotoxicity, and the NOAEC was determined to 34 ppm [1, 3]. In addition, the inhaled DEB mixture affected the action potential of peripheral nerves via decreased motor and sensory conduction and increased the amplitude of sensory action in SD rats exposed to 500 ppm for 18-weeks [12]. In addition, inhalation exposure to a DEB mixture reduces the levels of WBCs and lymphocytes [1, 6].
Among the results of several studies on the toxicity of DEB isomers, 1,2-DEB is known to depress the central nervous system, and 1,2-Diacetylbenzenes, are metabolites of 1,2-DEB, have shown clear peripheral neurotoxicity [14, 15]. An oral administration of 1,2-DEB to rats at a dose of 1,000 mg/kg caused liver weight gain and centrilobular hypertrophy related with inductioin of hepatic enzymes and resulted in increased thyroid weight and changes in thyroid hormone levels [1].
Regarding the health effect of workers, despite the fact that the DEB substance has light soluble in water and modulate vapor pressure, this substance can be volatile from water or soil to the atmosphere and exposed to workers as a route of inhalation [3]. Therefore, we conducted a 13-week inhalation toxicity study in accordance with the OECD Guidelines (TG 413) to confirm the toxicity of the DEB mixture. The results of this study indicated that the inhalation of 0, 40, 80, or 160 ppm DEB in Wistar rats did not result in toxicity. Despite the consideration of higher concentrations of exposure by references and the preliminary test, the maximum test concentration was adjusted due to the structure of the chamber and the characteristics of the test material. Hematological examination showed a significant increase in PT in the medium- and high-exposure groups; however, no dose-dependent changes in other parameters or organs related to blood clotting function were identified. In the histopathological analysis, some lesions were found in the heart, liver, lung, nasal cavity, pancreas, pituitary, testes, thyroid, and vagina, but these findings were considered to be incidental or spontaneous, as they represented low severity and frequency and were commonly observed in similarly aged rats as background lesions. Therefore, the NOAEC of DEB was 160 ppm under the study conditions. However, additional studies will be needed to investigate the toxic effect of DEB such as neurotoxicity.
We conducted 13-week inhalation toxicity studies with vaporized DEB at a maximum concentration of 160 ppm in male and female Wistar rats, in accordance with the GLP and OECD guidelines. No toxic effects were observed on body weight, food consumption, BALF, blood, or gross or histopathological analyses. Based on these results, the NOAEC for DEB was determined to be 160 ppm.