INTRODUCTION
Animals and their by-products offer a diverse range of medicinal options. They have been extensively utilized in traditional medicine for a significant period of time. They also serve as a source of medicinal substances in traditional medicine throughout the world [1, 2]. The term ‘zootherapy׳ was introduced to describe the treatment of human diseases using therapeutics obtained directly or indirectly from animals [2, 3]. The inaugural Pharmacopoeia Rossica released in 1778 featured 29 monographs detailing medications sourced from animals [4]. In Latin America, at least 584 animal species have been empirically chosen for medicinal purposes [5]. Traditional Chinese Medicine (TCM) is a time-honored healthcare system that integrates the use of diverse animal and plant species, with TCM texts documenting up to 823 animal species utilized within its practices [6-8].
Korean Medicine (KM) also has utilized a variety of animal-derived medicinal source including Gekko gecko (G. gecko) a member of the Gekkonidae family commonly found across Asia [9].
In Korean medicine, G. gecko has been used to treat tuberculosis, asthma-like symptoms, diabetes, and cancer. Modern pharmacological research has indicated that gecko and formulations containing it are effective against various ailments including malignant tumors, osteomyelitis, tuberculosis, and syrinx [10]. Recently, there has been growing medical interest in gecko, particularly due to its potent anti-tumor properties, notably against digestive system cancers such as esophageal cancer, gastric cancer, and liver cancer [11-13]. Active components extracted from gecko demonstrate a range of pharmacological activities.
G. gecko has been used as medicinal source in Asian traditional medicine to treat diverse diseases such as cancer, asthma, diabetes, and skin disease [14-18]. G. gecko extract (GGE) has anticancer, antiangiogenic, and antioxidant properties [12, 19, 20]. In addition, previous study revealed that GGE could attenuate allergic inflammation in the airway, and it contains melatonin, bioactive compound with antioxidant and anti-inflammatory effects [16]. However, the toxicity of GGE is unclear. The safety of GGE was evaluated through toxicity tests as it was considered that it could be used to treat asthma and cancer. This study conducted a single-dose toxicity test using GGE. Mortality rates, clinical symptoms, changes in body weight, hematological examinations, and necropsy results were then observed. The aim of this study was to report significant findings obtained from these observations.
MATERIALS AND METHODS
Dried G. gecko were obtained from Gwangmyeongdang (Ulsan, Korea). Following genetic and morphological analysis, a voucher specimen was deposited at the Korean Herbarium of Standard Herbal Resources (Voucher no. 2-18-0120) in the Korea Institute of Oriental Medicine. The bodies of G. gecko, weighing 750 g, were extracted twice with 10 L water for 3 hr. The extract was then concentrated under reduced pressure (yield: 17.46%) [16]. Before administration to rats, the extract of G. gecko was organoleptically identified as shown in Table 1.
Seven-week-old male and female Sprague-Dawley (SD) rats were obtained from Orientbio (Seongnam, Korea) and acclimated for six days. During acclimatization and experimental periods, animals were provided free access to food pellets (Teklad Certified Irradiated Global 18% Protein Rodent Diet, Envigo, Indianapolis, IN, USA) and tap water. Animals were housed at 22 ± 2°C with a relative humidity of 55 ± 10% and light/dark cycle (12:12 hr). This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Korea Conformity Laboratories (Incheon, Korea) based on Animal Protection Act of Korea (Approval No.: IA20-02696).
Animals (20 males and 20 females) were allocated to four experimental groups of GGE receiving 0, 500, 1,000, or 2,000 mg/kg.
After starvation for 4 hr, rats were orally administered with GGE at a dose of 500, 1,000, or 2,000 mg/kg or vehicle alone. The application volume (10 mL/kg) was calculated according to body weight on the treatment day. The intended clinical route for the test article is oral administration.
Gross appearances of animals were observed immediately and at 30 min, 1, 2, 3, 4, 5, and 6 hr after administration. Thereafter, they were observed once a day for 14 days. Rats were also observed once daily for mortality and morbidity for 14 days. Clinical signs such as respiration, skin, fur, gait, posture, response to handling, bizarre movements, stereotypy, convulsions, mucus, and eye/pupil were observed.
Individual body weights of rats were measured shortly before test article administration and at 1, 3, 7, and 14 day after treatment.
On day 14 after treatment, all surviving rats were weighted and then sacrificed by cutting the abdominal aorta with posterior vena cava under isoflurane anesthesia for macroscopic observation. These animals underwent gross necropsies such as examination of the outer body surface, thoracic cavity, abdominal cavity and cranial cavity, and their contents.
On the 14th day, rats were weighed, euthanized after anesthesia, and blood samples were collected for hematology analysis. Hematological parameters were examined by using an ADVIA 2120i hematology analyzer (Siemens Ireland, Dublin, Ireland). Hematological analysis included measurement of white blood cell (WBC) count, differential WBC count, hematocrit (HCT), red blood cell (RBC) count, hemoglobin (Hb), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), and platelet (PLT).
Weight changes and hematological results of the experimental groups and control group were analyzed for significance by one-way analysis of variance (ANOVA) using SPSS 12.0 K software (SPSS, Chicago, IL, USA). p-value<0.05 was considered to be significant. Results are expressed as mean ± standard deviation (S.D.).
RESULTS
Mortality and abnormalities in gross appearance of animals were not observed during the experimental period. During clinical observation, fur, skin, eyes, mucous, membrane, gait, posture and respiration appeared normal. Lacrimation, clonic or tonic movement, salivation, piloerection, diarrhea, stereotype, and bizarre behaviors were not observed either (Table 2).
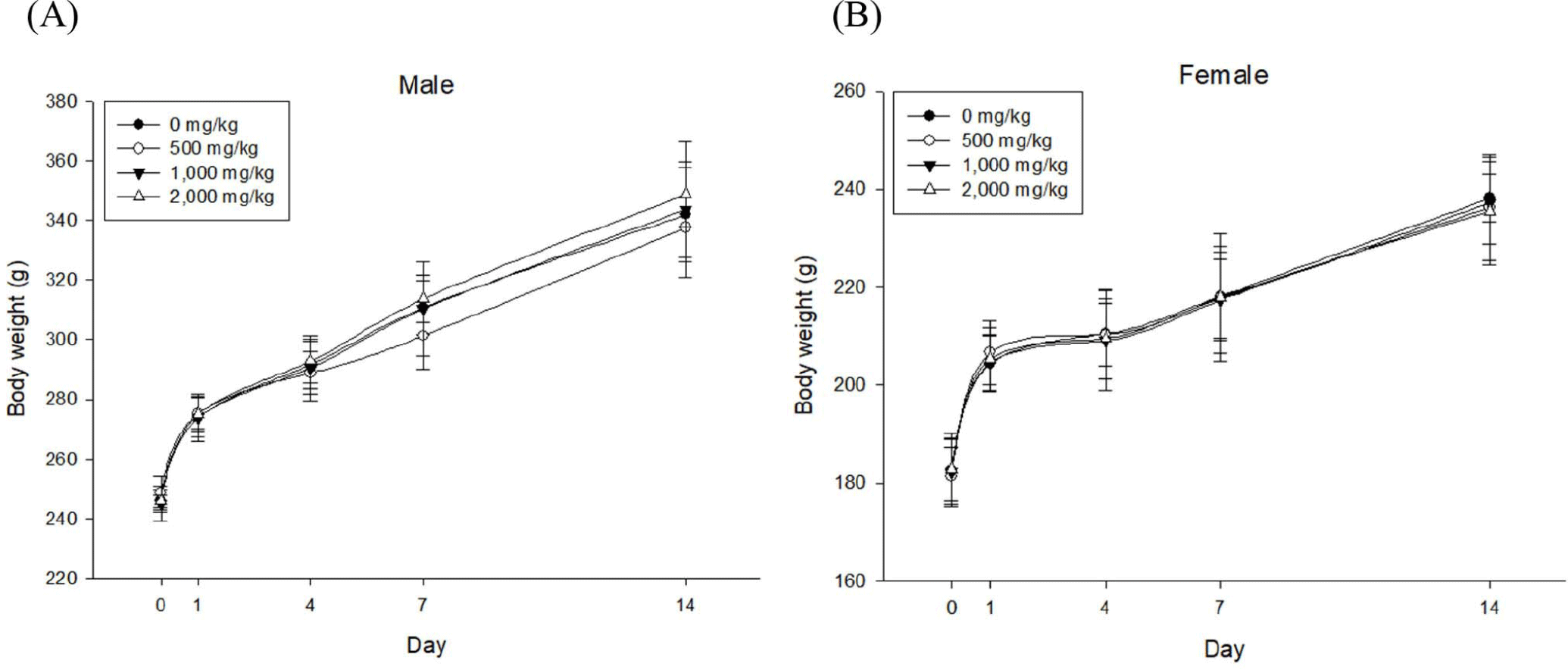
Body weight gains of male and female experimental groups (GGE 500, 1,000, or 2,000 mg/kg groups) were similar to those of their corresponding control groups, showing no significant (p>0.05) differences (Fig. 1).
No abnormal findings were observed in macroscopic observation on day 14 (Table 3).
Tables 4 and 5 show hematological parameters. Hematological parameters such as Hb, total RBCs, HCT, RBC indices, total and differential WBC count, and PLT count in GGE administrated animals were not significantly different from those of control animals.
WBC, white blood cell; NE, neutrophil; EO, eosinophil; BA, basophil; LY, lymphocyte; MO, monocyte; LUC, large unstained cell; NEP, percent of neutrophil; EOP, percent of eosinophil; BAP, percent of basophil; LYP, percent of lymphocyte; MOP, percent of monocyte; LUP, percent of large unstained cell; RBC, red blood cell; Hb, hemoglobin; RDW, red cell distribution width; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet; MPV, mean platelet volume.
WBC, white blood cell; NE, neutrophil; EO, eosinophil; BA, basophil; LY, lymphocyte; MO, monocyte; LUC, large unstained cell; NEP, percent of neutrophil; EOP, percent of eosinophil; BAP, percent of basophil; LYP, percent of lymphocyte; MOP, percent of monocyte; LUP, percent of large unstained cell; RBC, red blood cell; Hb, hemoglobin; RDW, red cell distribution width; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet; MPV, mean platelet volume.
DISCUSSION
Korean medicines are increasingly being used not only as medicinal resources, but also as major food resources. Among 514 types of herbal medicines managed as pharmaceuticals in Korea, 117 types are managed for both Korean medicine and food use [21, 22]. They can be purchased and used by the general public without special regulations. The use of these Korean medicines only relies on empirical usage and dosage with insufficient scientific evidence. Therefore, we believed that there should be continuous and systematic research on the safety of them.
G. gecko is one of a medicinal sources in Korean medicine. It has been used to treat asthma-like symptom due to its therapeutic effect of stopping coughs [16]. In recent literature, various efficacy effects of G. gecko are being scientifically proven and new research results are being published, attracting more attention. Studies on G. gecko’s anti-cancer effects have shown that G. gecko can inhibit proliferation of H22 and Bel-7402 liver cancer cells, HeLa cervical cancer cells, and EC-109 esophageal squamous carcinoma cells [17, 23, 24]. It can also inhibit angiogenesis [12, 25]. Based on these studies, G. gecko can be developed for use in various fields. However, sufficient research on safety of G. gecko has not yet been reported. Therefore, scientific verification of the safety of G. gecko is necessary.
In this study, mortality, clinical symptoms, weight changes, hematologic examinations, and macroscopic examinations were performed to evaluate single-dose toxicity to provide a clear basis for the safety results. In acute toxicity studies, we did not find any clinical changes produced in animal physiological or psychological symptoms. Body weight and hematological examination results were not significant compared to those of the control group either. Thus, LD50 of GGE was considered to be greater than 2,000 mg/kg.
Overall, these results indicate that GGE has no significant toxicity. However, a single oral dose acute toxicity test is not sufficient to determine toxicity of GGE. Further research should include 4- and 13-week repeated oral dose toxicity studies and genotoxicity studies. These studies are expected to provide more accurate and scientifically based safety data for establishing systematic toxicity information on GGE.