INTRODUCTION
Canine diabetes mellitus (DM) can be classified as insulin deficiency diabetes (IDD), resulting from a congenital deficiency or acquired loss of pancreatic beta (β) cells, or insulin resistance diabetes, resulting from concurrent insulin-antagonistic conditions [1, 2]. Canine DM is usually IDD, meaning that the loss of β-cells function is irreversible and requires lifelong insulin administration [1]. In dogs, unlike humans and cats, where obesity-induced insulin resistance diabetes is common, remission of diabetes is very rare; however, it can be achieved by resolving the underlying causes, such as estrus, pregnancy, hyperadrenocorticism (HAC), or administration of glucocorticoids [1, 3, 4].
Previous studies have reported cases of transient diabetes associated with insulin-resistance dogs. Hormone imbalance and inflammatory cytokines play major roles in the development of insulin resistance [5, 6]. Herein, we report a rare case of transient DM induced by cortisol and progesterone in a Schnauzer dog.
CASE REPORT
A 12-year-old intact female Schnauzer presented with polyuria, polydipsia, and lethargy at a local veterinary clinic. The biochemical analysis revealed remarkable hyperglycemia; however, the dog was non-responsive to insulin (Neutral Protamine Hagedorn insulin, 0.4 IU/kg, subcutaneous [SC], twice a day) for 2 weeks: blood glucose concentration was 473 mg/dL (reference range [RR], 70–143 mg/dL), indicating a decreased insulin sensitivity. After referral to the veterinary medical teaching hospital, tachypnea (respiratory rate, 40 breaths/min), systemic hypertension (Doppler blood pressure, 144 mmHg), and abdominal pain were observed. The abdomen was distended with a potbelly appearance, and comedones were observed on the dorsal trunk. The owner noticed that the last estrus cycle occurred nine months ago, and multiple mammary masses were observed 3 years ago.
Laboratory screening tests included complete blood count, biochemical analysis, urinalysis, and abdominal ultrasonography. Complete blood cell count revealed non-regenerative moderate anemia (packed cell volume, 28%; RR, 36%–55%), monocytosis (monocyte count, 2.25 × 103/μL; RR, 0.16–1.12 × 103/μL), and thrombocytosis (platelet count, 1,360 × 103/μL; RR, 148–484 × 103/μL). Abnormalities in the biochemical profile included increased levels of alkaline phosphatase (ALP, 304 IU/L; RR, 23–212 IU/L), hyperglycemia (459 mg/dL; RR, 70–143 mg/dL), elevated fructosamine (423 μmol/L; RR, 177–314 μmol/L), hyperglobulinemia (4.6 g/dL; RR, 2.5–4.5 g/dL), hypercholesterolemia (358 mg/dL; RR, 110–320 mg/dL), and hypertriglyceridemia (271 mg/dL; RR, 10–100 mg/dL). Blood β-hydroxybutyrate measured with a portable ketone monitor (FreeStyle Optium, Abbott, Chicago, IL, USA) was elevated (4.7 mmol/L; RR < 0.6 mmol/L), but metabolic acidosis was not observed (pH 7.38; RR, 7.35–7.45). Urinalysis revealed a urine-specific gravity of 1.034, glycosuria (> 100 mg/dL), ketonuria (150 mg/dL), and a pH of 5.0 (RR, 6.0–7.5). Persistent hyperglycemia with ketosis was obvious, but abdominal pain, stress leukogram, and increased ALP indicated that the dog might have concurrent HAC or pancreatitis. The estrous cycle was also included in the differential diagnosis of insulin resistance.
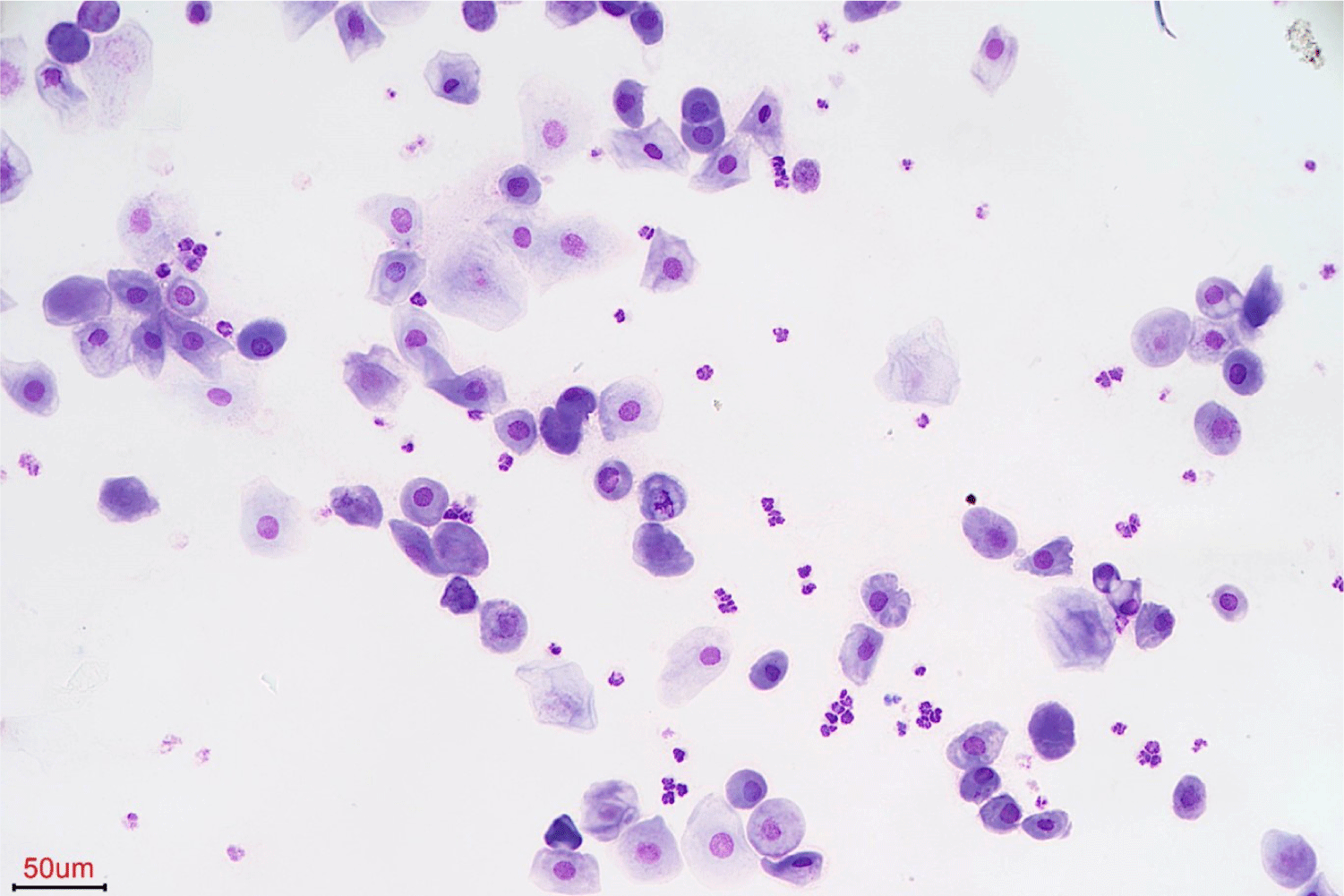
To determine the presence of insulin resistance, a low-dose dexamethasone stimulation test (LDDST), canine pancreatic lipase immunoreactivity assay (SNAP cPL; IDEXX Laboratories, Westbrook, ME, USA), and vaginal smear cytology were performed. Cortisol levels at 4 and 8 h after LDDST were above the laboratory cutoff (> 1.5 μg/dL; IDEXX Laboratories), thus establishing a diagnosis of HAC. Pancreatitis was confirmed based on abnormal SNAP cPL test results. A vaginal smear indicated diestrus with predominantly intermediate epithelial cells and several leukocytes (Fig. 1). Additional abdominal ultrasonography revealed hyperechoic changes in the hepatic parenchyma and bilateral enlargement of the adrenal glands.
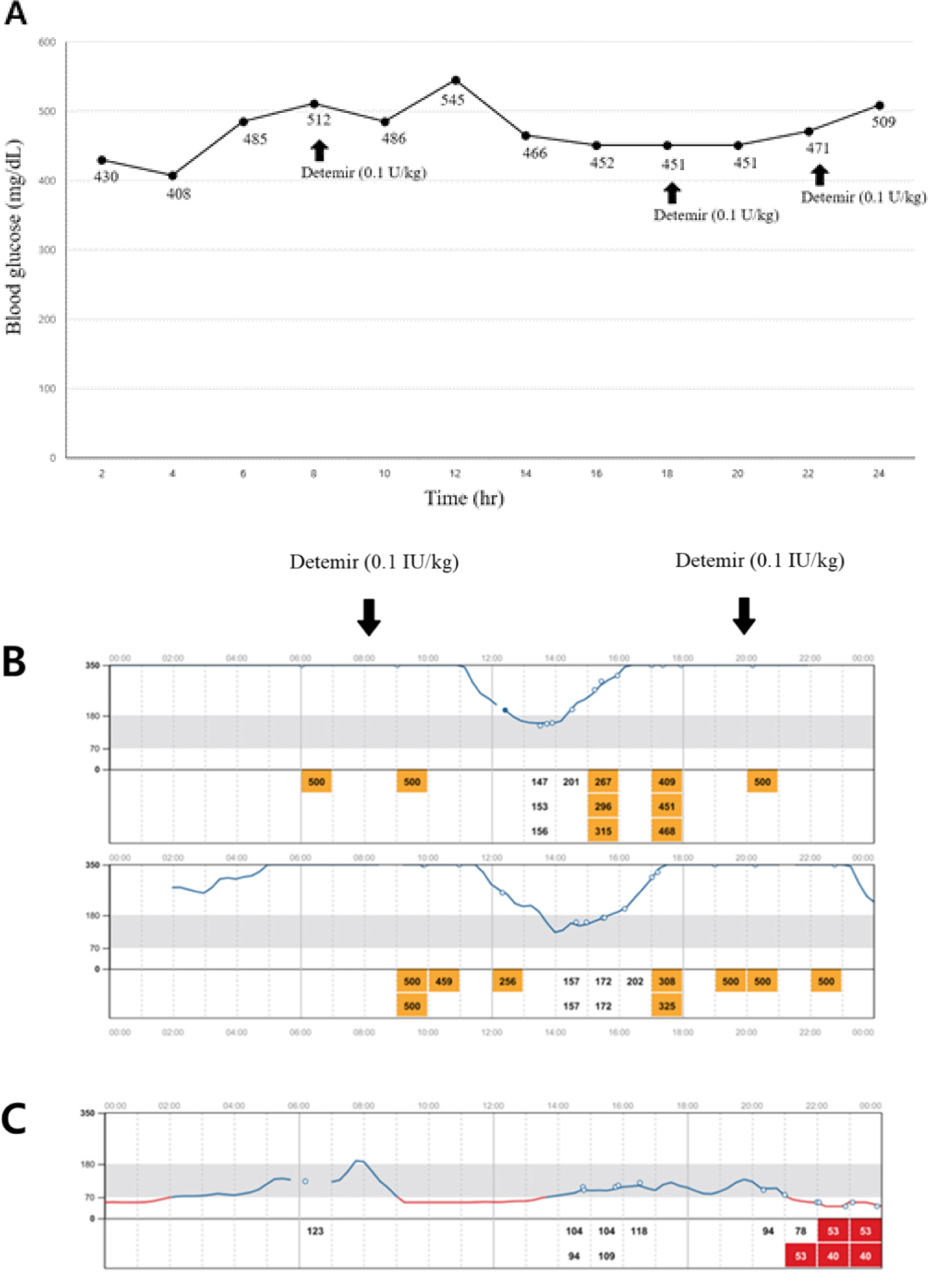
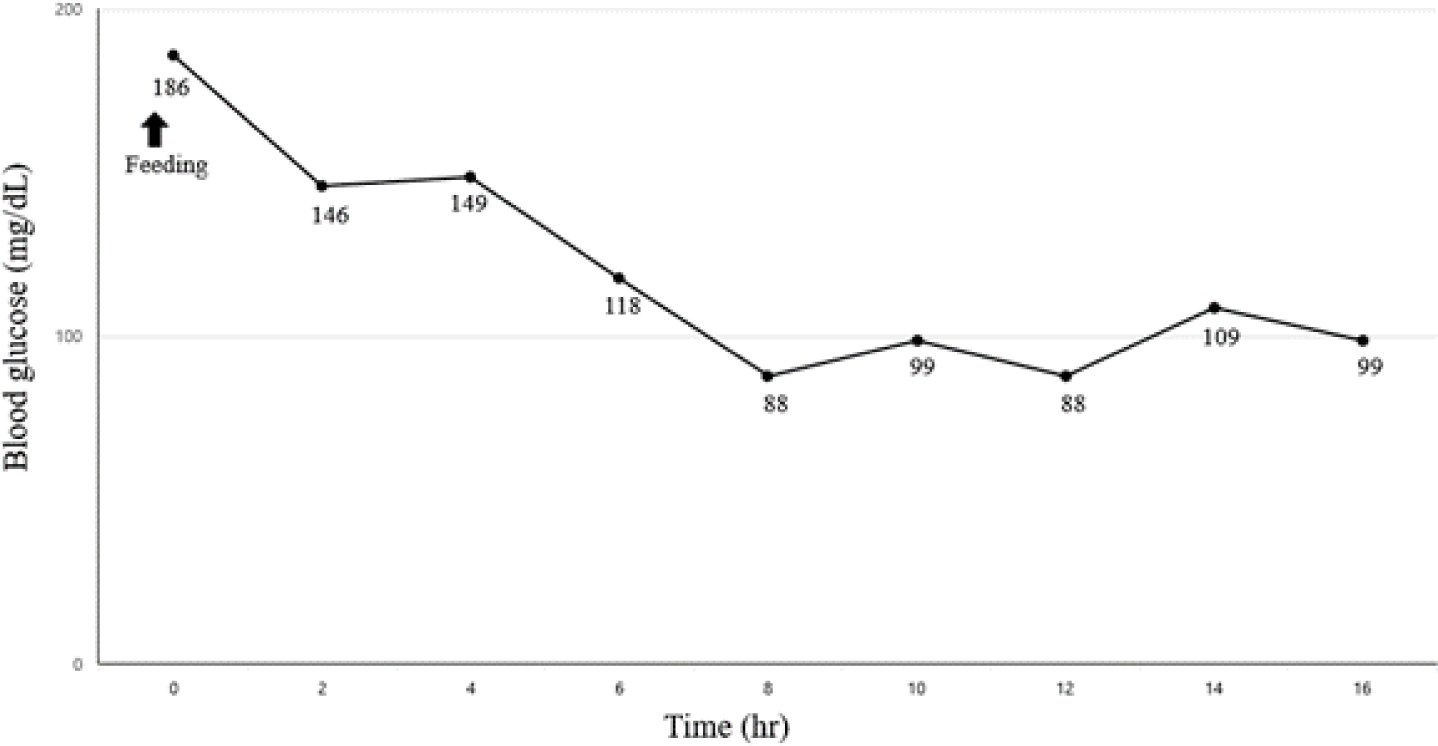
Considering a possible insulin resistance, blood glucose levels were monitored using a flash glucose monitoring system (FGMS; FreeStyle Libre, Abbott Diabetes Care, Alameda, CA, USA) for 2 weeks; insulin therapy was initiated using detemir (0.1 IU/kg, SC, twice a day). Despite insulin administration, the blood glucose level remained consistently high, more than 400 mg/dL (RR, 70–143 mg/dL) during 7 days after initiating the treatment (Fig. 2A). Starting on day 7, the dog was administered trilostane (1 mg/kg every 12 h) for treating HAC. On day 14, attaining control of HAC, blood glucose level gradually declined to a nadir of less than 200 mg/dL (RR, 70–143 mg/dL; Fig. 2B), and ketosis had improved (β-hydroxybutyrate, 2.1 mmol/L; RR < 0.6 mmol/L). However, the glycemic control remained poor, with the highest glucose concentration being above 500 mg/dL and a short duration of the insulin effect. Eventually, the dog underwent ovariohysterectomy (OHE) and complete mastectomy to improve insulin sensitivity. One week after surgery, the blood glucose level (mean blood glucose < 85 mg/dL) was controlled and insulin therapy was discontinued (Fig. 2C). One month later, the blood glucose curve was well-controlled, with a mean blood glucose level of 120 mg/dL (RR, 70–143 mg/dL; Fig. 3), and no glucosuria was observed. The dog remained in remission for approximately 4 months, but eventually the clinical signs of polyuria and polydipsia were relapsed. To determine the main cause of diabetes recurrence, biochemical analysis, serum progesterone concentration, adrenocorticotropic hormone stimulation test (ACTHST), canine pancreatic lipase immunoreactivity assay (Spec cPL; IDEXX Laboratories), and abdominal ultrasonography were performed. The progesterone concentration was less than 0.2 ng/mL, and the dog was considered to be in anestrus (laboratory cutoff for anestrus < 1.0 ng/mL; IDEXX Laboratories). Post-ACTHST serum cortisol concentration was 3.8 μg/dL (RR, 1.5–6.0 μg/dL) indicating that HAC was being well controlled. Biochemical analysis revealed hypercholesterolemia (338 mg/dL; RR, 110–320 mg/dL), hypertriglyceridemia (> 375 mg/dL; RR, 10–100 mg/dL), and elevated Spec cPL (253 μg/dL; RR, 0–200 μg/dL). Compared with prior findings, diagnostic imaging revealed hyperechoic changes in the pancreatic parenchyma. Hence, we assumed that the progression of pancreatitis associated with persistent hyperlipidemia was a direct cause of diabetes recurrence and that the condition was permanent. The dog was well-responsive to insulin injection, and well-managed at present.
DISCUSSION
In this case report, a dog developed diabetic ketosis due to insulin resistance, which was remitted by diagnosing the cause of insulin resistance using an FGMS and eliminating each underlying condition appropriately. The dog presented with persistent hyperglycemia, which could not be controlled with the therapeutic dosage of insulin. Based on the findings of a vaginal smear, the dog was considered to be in diestrus. Diestrus in intact females and HAC are the two main causes of insulin resistance in dogs [7], and after the management of HAC and OHE, the dog achieved diabetes remission. Cases of diabetes remission after eliminating the causes of insulin resistance indicate that β-cells are not completely impaired and, therefore, normal glucose levels can be maintained [6].
During the estrous cycle, a normal female dog, regardless of pregnancy, maintains a diestrus in which progesterone is released. In dogs, progesterone stimulates increased secretion of growth hormone (GH) by the mammary glands, and both hormones can decrease tissue sensitivity to insulin [5, 6, 8]. Previous studies have analyzed fat cell cultures and demonstrated that progesterone inhibits the binding of insulin to the insulin receptor, and also that GH modulates insulin sensitivity through several intracellular mechanisms [9, 10]. Several studies have reported on female dogs that develop diabetes shortly after estrus [11, 12]. Although progesterone-mediated insulin resistance is well characterized, case reports correlating canine diabetes with diestrus, mainly regarding its remission after spaying, are scarce [6, 13]. Recently, a study reported a relationship between diestrus, gestation, and diabetes in 63 Elkhounds diagnosed with diabetes and a high incidence of diabetes in dogs with late estrus and pregnancy [5]. In addition, a retrospective study investigated the remission rate of canine DM after the resolution of complications associated with ovarian activity; of 117 female dogs diagnosed with canine DM, six achieved remission: five after OHE, and one at the end of diestrus [6]. According to these studies, the chance of remission increases when OHE is performed as soon as possible following the development and diagnosis of DM; in this case, OHE was performed within 2 weeks of diagnosis, and remission was achieved [1, 5, 6].
HAC may also be a risk factor for diabetes. Glucocorticoids antagonize the effects of insulin, inducing a sustained increase in blood glucose levels, and in the most severe cases, the development of DM [3, 4, 14, 15]. When analyzing the risk of developing DM in dogs with HAC, those with fasting blood glucose levels > 100 mg/dL, dyslipidemia (either high triglyceride levels > 250 mg/dL, or hypercholesterolemia > 350 mg/dL) and intact females are more likely to develop diabetes; in this case, the dog met all of the above criteria at the initial diagnosis [14]. After the dog was started on trilostane to manage the HAC, the need for insulin decreased. However, this was not considered to play a major role in DM remission. Diabetes remission following resolution of HAC has not been reported in dogs, and in humans with both diabetes and HAC, resolution of HAC is not associated with diabetes remission [15].
In addition to hormones, several other factors can contribute to the development of DM in dogs, including inflammation, hyperlipidemia, overweight, lack of physical activity, and an unbalanced diet [1, 6, 16]. Hyperlipidemia and pancreatitis, common concurrent conditions associated with diabetes, were observed at the time of diagnosis. Miniature Schnauzer dogs are predisposed to primary hyperlipidemia, which increases the risk of pancreatitis [17–19]. In a recent study, almost 30% of Miniature Schnauzers with primary hypertriglyceridemia showed evidence of insulin resistance, as determined by serum insulin concentration [19]. Pancreatitis is also closely related to diabetes, and it has been proposed that pancreatitis may lead to DM via “bystander” β-cell damage, resulting in the release of protein antigens usually “hidden” from the immune system, and initiation of β-cell autoreactivity [20]. In this dog, diabetes remission was achieved after OHE; however, it permanently relapsed 4 months later. It is assumed that the β-cells were still functional at the time of surgery, but irreversible β-cells damage had occurred due to progressive pancreatic atrophy, gradually leading to insulin-deficient DM. Even after relapse, the dog had erratic glycemic control and the insulin requirements fluctuated constantly, which was thought to be caused by decreased insulin sensitivity resulting from pancreatitis and hyperlipidemia.
This case had some limitations due to the inability to measure blood progesterone and C-peptide levels at the first visit to demonstrate diestrus and the subsequent development of insulin-resistant diabetes. Here, we highlight the importance of accurately diagnosing and managing comorbidities in dogs with suspected insulin resistance.