INTRODUCTION
Collagen serves as a fundamental component in connective tissues, tendons, bones, teeth, and the cornea, providing essential structural support to the bodies of all vertebrates [1]. Due to its exceptional biocompatibility, minimal antigenic reactivity, significant biodegradability, hemostatic properties, and cellular binding affinity, collagen has become a ubiquitous biomaterial across various fields including medicine, dentistry, pharmacology, cosmetology, and tissue engineering [2–5]. Notably, collagen-derived peptides have emerged as a key ingredient in functional foods, in addition to garnering widespread attention from numerous research fields due to their ability to regulate physiological and hormonal functions [6]. However, the existing knowledge regarding the toxicity of these widely used functional food constituents remains insufficient.
Collagen peptides can be obtained from various origins including animals, plants, and marine organisms. Among these, marine-derived collagen peptides, valued for their economic viability and efficient absorption due to their reduced molecular weight, hold particular significance [7]. The exploration of biological applications and effectiveness of marine collagen peptides encompasses various sources such as salmon, tilapia, shark, and jellyfish [8]. Collagen peptides derived from marine fish have been acknowledged as dietary supplements with potential benefits for regulating blood pressure [9], managing glucose levels [10], enhancing skin hydration [11], and improving lipid profiles [12]. Particularly, collagen peptides sourced from skate (Raja kenojei) skin (CPSS) are highly valued for their potential contributions to anti-obesity strategies, reduction of oxidative stress, and mitigation of inflammation [13, 14]. Skate skin contains substantial levels of bioactive compounds such as taurine, which plays a key role in cellular growth and energy enhancement, as well as anserine, a muscle buffer [15]. However, previous studies have not comprehensively assessed the toxicity of specific CPSS-derived collagen peptides. Therefore, the extensive use of collagen peptides as components in functional foods highlights the need to establish comprehensive safety data for these substances.
In this study, we conducted a comprehensive assessment of the toxicity of collagen peptides derived from CPSS through single-dose oral administration in both rats and dogs. The design and execution of the rat and dog studies strictly followed the guidelines set forth by the Organisation for Economic Co-operation and Development (OECD) and the Korea Ministry of Food and Drug Safety (KFDA), operating within the framework of modern Good Laboratory Practice regulations. Our findings thus provide a theoretical basis for the selection of CPSS doses for an upcoming 90-day repeat-dose toxicity investigation [16], as well as for future clinical trials to explore the potential of CPSS as a promising dietary supplement for human consumption.
MATERIALS AND METHODS
The methodologies for preparing CPSS and subsequent HPLC analysis closely followed the established procedures outlined in our previous publication [16]. The initial material was obtained from frozen skate (R. kenojei) skin samples. High-pressure extraction was conducted by subjecting the samples to a 121°C temperature for 1.5 h. Following this extraction step, the skin samples were rapidly frozen at −50°C for 2 h. Next, the samples underwent a 2-h hydrolysis process using a combination of 0.1% alcalase (Novozymes, Bagsværd, Denmark) and flavorzyme (Novozymes). Deodorization was carried out for 30 min at 75°C using activated carbon. The resulting mixture was then filtered and concentrated at 60°C under reduced pressure. The final product (CPSS) was obtained in a freeze-dried format, serving as the primary raw material.
Chromatographic separations were conducted using an HPLC-20A system (Shimadzu, Kyoto, Japan) equipped with a photodiode array detector. The separation was achieved using a ZORBAX Eclipse Plus C18 column (250 × 4.6 mm, 5 μm, Agilent, Santa Clara, CA, USA) at an operational temperature of 30°C, with a detection wavelength of 250 nm, an injection volume of 10 μL, and a flow rate of 1.0 mL/min. The mobile phase, composed of a 20:80 v/v ratio of 0.1% aqueous formic acid (aq.) to methanol, was effectively filtered using a 0.45 μm polytetrafluoroethylene membrane filter. The chromatographic resolution of H-glycine-proline-hydroxyproline (GPH)-OH was achieved within 5 min. The concentration of H-GPH-OH in the samples was quantified by comparing calibration curves. To ensure precision, HPLC measurements were performed in triplicate to confirm and adjust the concentration of H-GPH-OH, maintaining a consistent content of 8 mg per gram of sample.
A single oral toxicity study was conducted using female Sprague-Dawley rats aged 9 to 10 weeks. The rats were obtained from Orient Bio (Gapyeong, Korea) and were allowed to acclimate to their new environment for 1 to 2 weeks before being included in the study. Throughout our study, these animals were housed in stainless steel wire cages (dimensions: W270 × L500 × H200 mm) within a controlled environment. This controlled environment featured regulated light-dark cycles (12–12 h), a controlled air exchange rate (10–20 changes/h), consistent temperature levels (20°C–23°C), and constant relative humidity (48%–59%). During the study, the rats were given ad libitum access to radiation-treated commercial rat chow (Purina Korea, Gunsan, Korea) and ultraviolet-sterilized tap water. All experimental protocols involving animal tests were approved by the Institutional Animal Care and Use Committee of the Korea Testing and Research Institute (KTR IACUC ID: IAC2020-2578).
A single oral dose escalating toxicity study was conducted using beagle dogs of both sexes, all aged 6 months. The dogs were sourced from Jiansu Johnsen BioResource (Jiangsu, China). A thorough acclimatization period of 21 days was employed within a controlled environment prior to conducting the experiments. This environment was meticulously maintained with a temperature range of 21.5°C–23.0°C, a relative humidity range of 47.0%–51.6%, air circulation occurring at a frequency of 10–20 changes per hour, and artificial lighting maintained at 150–300 lux from 8 am to 8 pm. Throughout this acclimatization phase and the study itself, the dogs were accommodated within stainless-steel wire cages measuring 900 (W) × 850 (L) × 800 (H) mm. Throughout the experiment, the dogs were granted ad libitum access to laboratory animal feed pellets (5LL9, Gapyeong, Korea) and filtered water. All experimental procedures involving these animals were scrutinized and approved by the Institutional Animal Care and Use Committee of the Korea Testing and Research Institute (KTR IACUC ID: IAC2020-2911).
After a week of acclimation and a one-week quarantine period, six healthy female rats selected for this study were randomly assigned to two experimental groups: one receiving 300 mg CPSS/kg body weight in the Step 1 (n = 3) and another receiving 300 mg CPSS/kg body weight in the Step 2 (n = 3). Additionally, following an extended period of acclimation and quarantine lasting two weeks, six healthy female rats were randomly assigned to two separate experimental groups: one receiving 2,000 mg CPSS/kg body weight in the Step 3 (n = 3) and another receiving 2,000 mg CPSS/kg body weight in the Step 4 (n = 3) of CPSS. The selected dosage levels were in line with the recommendations outlined in the OECD test guidelines [17]. CPSS was administered via gavage in a volume of 10 mL/kg body weight after overnight fasting (approximately 18 h; water was not restricted). The interval between CPSS administrations was carefully selected based on observed clinical signs and their severity. Following the initial administration of CPSS to three animals at a dose of 300 mg/kg body weight, subsequent dosing steps were conducted according to established guidelines.
Healthy adult male and female dogs (n = 1 in the control group, n = 2 in the treatment group) were randomly assigned to two distinct experimental groups: a treatment group (with dose escalation cohorts of 500, 1,000, and 2,000 mg/kg of CPSS with 3-day intervals) and a control group. The selection of these dosage levels was in accordance with the recommendations outlined in the KFDA guidelines (2017-71, 2020-63). CPSS was administered via gavage in a volume equivalent to 5 mL/kg body weight after overnight fasting (approximately 18 h; water was not restricted).
Throughout the study, all animals underwent daily observations to monitor potential clinical signs of toxicity and mortality. This monitoring was conducted for 14 days after CPSS administration. Additionally, all experimental animals were observed at 0.5, 1, 2, 3, and 4 h post-administration.
In the single oral toxicity study, the body weights of each rat were measured immediately prior to the initiation of CPSS administration and on days 1, 3, 7, and 14 post-administration. For the single oral dose escalating toxicity studies, the body weights of each dog were measured immediately before the start of each CPSS administration, on the day after each administration, as well as on days 1, 3, 7, and 14 following the final administration.
On the scheduled necropsy day, both rats and dogs were subjected to an overnight fasting period followed by anesthesia administration. Rats were anesthetized using inhaled isoflurane (Hana Pharm, Seoul, Korea), while dogs received anesthesia through intravascular injections of ketamine (Yuhan, Seoul, Korea) and were subsequently euthanized with intravascular injections of T61 (Intervet, Seoul, Korea). During the anesthesia, necropsy procedures were conducted on each animal. All major organs and tissues within the abdominal and thoracic cavities were then examined to identify any evident gross lesions or abnormalities. Following the necropsy and the documentation of any macroscopic abnormalities, all animals were appropriately disposed of.
RESULTS
The rats consistently demonstrated good health and activity throughout the study, without any instances of morbidity or mortality, as indicated in Table 1. Particularly, the estimated LD50 (lethal dose for 50% lethality) resulting from CPSS administration exceeded 5,000 mg/kg for rats. Additionally, the animals exhibited no noticeable abnormalities in terms of fur condition or behavior. Moreover, there were no indications of urinary or gastrointestinal disturbances, such as hematuria, hematochezia, or diarrhea. Over the course of the 14-day period, there were no significant differences in body weight changes observed across each dose step, and all treated animals exhibited consistent and healthy weight gains over time (Table 2).
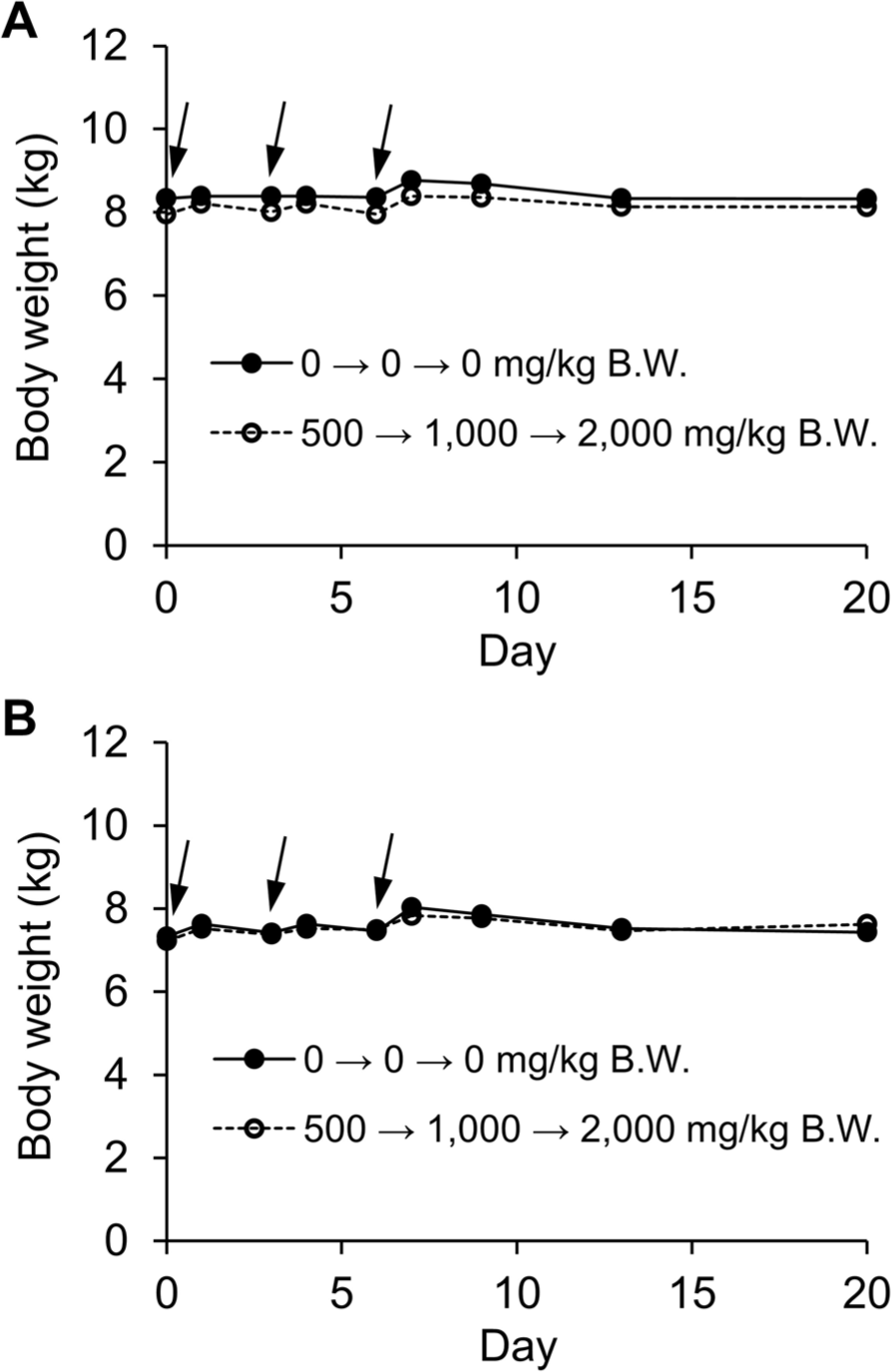
The dogs consistently maintained robust health and sustained a high level of activity throughout the duration of the study. Importantly, no instances of morbidity or mortality were observed among the canine subjects, as indicated in Table 3. Significantly, the values of the maximum tolerated dose (MTD) resulting from CPSS administration to dogs greatly exceeded the threshold of 2,000 mg/kg. Furthermore, there were no observable abnormalities concerning their fur condition or behavior. Additionally, there were no indications of disruptions in urinary or gastrointestinal functions, including occurrences of hematuria, hematochezia, vomiting, or diarrhea. Over the span of the 21-day treatment period, there were no statistically significant differences in the body weights of both male and female subjects treated with the test substance when compared to the control group (Fig. 1).
Upon concluding the clinical observation period, all animals were euthanized and underwent autopsy. A thorough examination was conducted on all major organs and tissues, including those within the abdominal and thoracic cavities, such as the lung, heart, thymus, liver, stomach, spleen, intestine, kidney, adrenal gland, pancreas, and reproductive organs. Remarkably, in both rats and dogs, no pathological irregularities or lesions were detected regardless of the administered CPSS dosage. A comprehensive overview of these findings is provided in Table 4.
DISCUSSION
Collagen peptides have garnered significant attention due to their potential range of benefits, including anti-aging properties [5, 18], antioxidant effects [19, 20], potential contributions to anti-obesity strategies [21], mitigation of alcohol intoxication [22], promotion of wound healing [23, 24], cosmetic applications [25], and even medicinal uses [26]. Despite these promising aspects, uncertainties remain concerning the potential adverse impacts of collagen peptides on human health. Therefore, our study comprehensively investigated the acute toxicity of CPSS in both rat and dog subjects, utilizing single oral dosing to elucidate the broader toxicological landscape. The key aspects explored in this study included gross toxicity, mortality rates, LD50, and MTD.
Our findings revealed no instances of mortality in rats or dogs treated with CPSS. Throughout the study period, no discernible clinical signs indicative of abnormalities were noted in either rats or dogs, regardless of the administered dosage levels. Furthermore, no significant fluctuations in body weight, a critical physiological parameter, were observed between the treatment and control groups in both rat and dog subjects. Notably, these body weight dynamics align well with established patterns of body weight changes observed in normal rat and dog populations, as evidenced by previous research [27, 28].
In contrast with a previous study where gelatin-containing collagen led to adverse effects such as taste issues, heartburn, and belching [29], the present investigation revealed no negative impacts on appetite, weight gain, or gastrointestinal functions due to oral CPSS administration in rats and dogs. Furthermore, the examination of major organs and tissues, including those within the abdominal and thoracic cavities, consistently indicated the absence of abnormal lesions in both rat and dog subjects, regardless of the administered CPSS dosage.
In conclusion, our findings demonstrated that acute oral exposure to CPSS is not associated with significant adverse effects in rats and dogs. The estimated LD and MTD values for CPSS administration were notably higher than 5,000 mg/kg (LD50) for rats and over 2,000 mg/kg (MTD) for dogs, respectively. The findings provide insights into the acute toxicity, LD50, and MTD of orally administered CPSS, thus enhancing risk assessment. Additionally, our findings provide a valuable basis for the use of CPSS in functional foods and medicinal applications.