INTRODUCTION
Babesiosis is a tick-borne disease caused by intraerythrocytic protozoa belonging to the genus Babesia transmitted by genera Dermacentor, Haemaphysalis, Hyalomma, Ixodes and Rhipicephalus ticks [1]. The disease is highly pathogenic to ruminants, horses, pigs, dogs, cats, and in some cases, even humans [2]. A babesiosis infection may be asymptomatic, but symptoms may also be acute, including fever, anemia, hemoglobinuria, and potentially death in severe cases. The severity of the illness depends on the patient’s immune status and the species of Babesia implicated [3, 4].
There are more than 100 different species of Babesia, some of which are zoonotic pathogens [5]. Babesia microti and Babesia divergens are known to infect humans as well as rodents and cattle [6, 7]. Wild animals are also known vectors of Ixodes spp. ticks [5, 8].
Wild cervids are often infected with some variety of Babesia spp., among which are some species that can infect humans. Reports from Europe indicate that B. divergens, Babesia capreoli and Babesia venatorum have been transmitted through wild cervids [9, 10]. B. capreoli is so similar to B. divergens that it was previously identified as B. divergens or B. divergens-like [11, 12]. Phylogenetic analysis is required to identify B. capreoli; in 2010 Malandrin et al. identified B. capreoli using three nucleotide difference positions at the 18S rRNA gene [13].
Despite its threat to animal and public health, babesiosis has rarely been studied in the Republic of Korea (ROK). In the present study, we report the identification of B. capreoli in engorged Ixodes nipponensis obtained from a Korean water deer (Hydropotes inermis argyropus).
MATERIALS AND METHODS
In the fall of 2018 a Korean water deer killed by a moving vehicle on a road in Jeollabuk-do was brought to the Jeonbuk Wildlife Rescue Center. An engorged female tick obtained from the deer was sent to the Animal Immunology Laboratory. The tick species was determined using primers for tick identification (Table 1). Blood and tissue samples were not collected from the Korean water deer.
| Target pathogen | Sequence (5′ to 3′) | Annealing (°C) | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| Anaplasma spp. |
TACCTCTGTGTTGTAGCTAACGC CTTGCGACATTGCAACCTATTGT |
58 | 429 | [14] |
| Babesia spp. |
GTTTCTGMCCCATCAGCTTGAC CAAGACAAAAGTCTGCTTGAAAC |
61 | 420–440 | [15] |
| Ehrlichia spp. |
CGGAATTCCTAGTGTAGAGG AGGAGGGATACGACCTTCAT |
58 | 340 | [14] |
| Rickettsia spp. |
TAGGGGATGATGGAATTCCTA CCCCCGTCA ATTCCTTTGAG |
58 | 252 | [14] |
| Theileria orientalis |
CACGCTATGTTGTCCAAGAG |
55 | 830 | [16] |
| Tick species |
CTGCTCAATGATTTTTTAAATTGCTGTGG CCGGTCTGAACTCAGATCAAGTA |
55 | 475 | [17] |
The engorged frozen tick was cut into pieces and the DNA was isolated using DNeasy Blood & Tissue Kits (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. Tick-borne pathogens, including species of Anaplasma, Babesia, Ehrlichia, Rickettsia, and Theileria, were screened via polymerase chain reaction (PCR) amplification and partial 18S rRNA gene sequence comparisons (Table 1). A negative control was included in the PCR assay in all experiments. PCR products were separated by electrophoresis on 1.5% agarose gel and visualized after ethidium bromide staining. The PCR products were purified using an AccuPrep® PCR Purification Kit (Bioneer, Daejeon, Korea) in accordance with the manufacturer’s instructions, after which they were directly sequenced (Macrogen, Seoul, Korea).
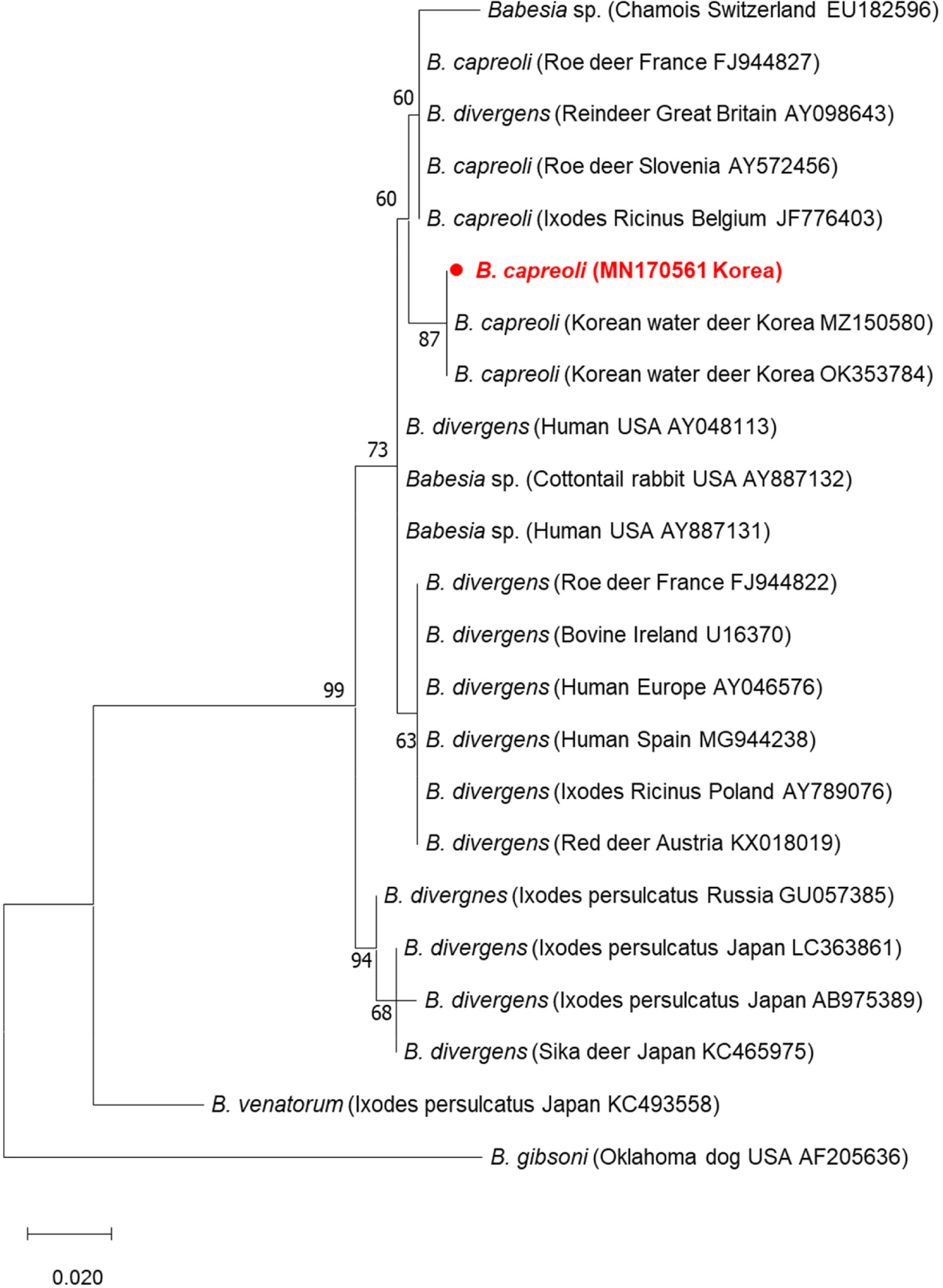
The nucleotide sequences thereby obtained were analyzed using BioEdit software (version 7.2.5) as well as the Basic Local Alignment Search Tool available from the National Center for Biotechnology Information database. To investigate the homologies of the Babesia gene, nucleotide sequences were aligned using ClustalX program and analyzed by direct comparison to reference sequences obtained from GenBank. Phylogenetic analysis based on B. capreoli partial18S rRNA gene sequences (356 bp). A tree was constructed using the MEGA7 software following the maximum likelihood method with Jukes-Cantor model (G+I). A bootstrap analysis was conducted with 1,000 replicates using MEGA7 software [18].
RESULTS
One adult tick obtained from the Korean water deer was identified with PCR analysis as I. nipponensis (Japanese hard tick). I. nipponensis is common throughout the ROK. Of the tick-borne pathogens tested for, only Babesia spp. was detected in the I. nipponensis. The B. capreoli infection was confirmed via PCR amplification and partial 18S rRNA gene sequence comparisons. The nucleotide sequence obtained in this study was ultimately submitted to the GenBank database with accession number MN170561.
Phylogenetic analysis based on the 18S rRNA gene sequences showed that the isolate found in I. nipponensis belonged to the B. capreoli lineage and was distinct from the Asian, European, and North American B. divergens lineages (Fig. 1). The B. capreoli lineage includes B. capreoli, Babesia sp., and B. divergens. According to a previous sequence analysis, B. capreoli is highly similar to B. divergens, with the sequence showing 99.83% homology. More specifically, the nucleotides at positions 631, 663, and 1637 in the 18S rRNA gene are different between the two species [19]; B. capreoli has G, T, and T, respectively, whereas B. divergens has A, A, and C. Knowing this, we were able to differentiate the two species. Two B. divergens (AY098643 and AY572456) and Babesia sp. (EU182596) isolates were confirmed to belong to the B. capreoli lineage, as opposed to B. divergens, as the nucleotides at these positions were identical to those of B. capreoli. Our sequence analysis revealed that our isolate shows 98.86%–99.47% homology with the B. capreoli lineage. And our sequence had 96.6%–99.6% identity to those of B. capreoli reported from Korea (Fig. 1). This may be due to a different tick species having transmitted the B. capreoli or a different host species.
DISCUSSION
We report a case of B. capreoli infection from an I. nipponensis tick in the ROK. I. nipponensis larvae and nymphs are found only among small mammals, whereas adults feed on larger animals; moreover, nymphs and adults frequently bite humans [20]. I. nipponensis is known to transmit potential zoonotic tick-borne pathogens in the ROK [20–22]. Although the present study does not confirm whether the Korean water deer was infected with B. capreoli, we cannot rule out this possibility. One recent study suggested that the Korean water deer as act as a reservoir host for B. capreoli [23]. As we were unable to identify the reservoir host for the B. capreoli transmission, the existence a of B. capreoli infection in the ROK warrants further study.
In Europe, Ixodes ricinus is the main vector for B. microti, B. divergens, B. venatorum, and B. capreoli [24], whereas in the present study, I. nipponensis is suggested as being responsible for the transmission of B. capreoli in the ROK. Although we cannot draw a definite conclusion from this study alone, it is possible that regional ticks are the primary vectors for B. capreoli. The most dominant tick species in Korea are known as Haemaphysalis longicornis and Haemaphysalis flava, and some reports have identified Babesia spp. infection by H. longicornis in humans and cattle [25, 26], so further studies will be needed to identify Babesia spp. infection in these ticks.
In general, Babesia infections are usually made in Korean grazing cattle, though Babesia spp. are found in a wide range of hosts, such as rodents, cottontail rabbits, small mammals, cattle, and even birds [27]. We hypothesize that small rodents act as competent reservoirs, promote the maintenance of this parasite, and allow transmission to the next generation of feeding ticks. In addition, unlike domestic animals, the wide range of activity of wild animals allows the presence of diseases to spread to all regions, rather than being confined to one region. With this in mind, it may be worthwhile to further examine the life cycle and hosts of B. capreoli in the ROK through investigations of prevalence and pathogenicity in wildlife as a first step towards understanding the possible effects of B. capreoli on livestock and humans.