INTRODUCTION
Testicular germ cell tumors (GCT) arise from embryonic or extraembryonic differentiated totipotential germ cells. The most common types of GCT are classified by two major groups: seminomatous tumors, which are composed of cells that resemble primordial germ cells or early gonocytes, and nonseminomatous tumors, which may be composed of undifferentiated cells that resemble embryonic stem cells, as in the case of embryonal carcinoma, but the malignant cells may also differentiate along other lineages, generating yolk sac tumors, choriocarcinomas, and teratomas. Mixed GCT is composed of more than one GCT component including one or more nonseminomatous elements in a tumor. They account for about one-third of GCT and 69% of all nonseminomatous GCT [1]. We experienced two distinct GCTs in one testis, adjacent to each other. We considered this tumor to be a collision tumor, not mixed GCT. To our best knowledge, this is the first case report of a collision tumor including two distinct primary GCTs.
CASE REPORT
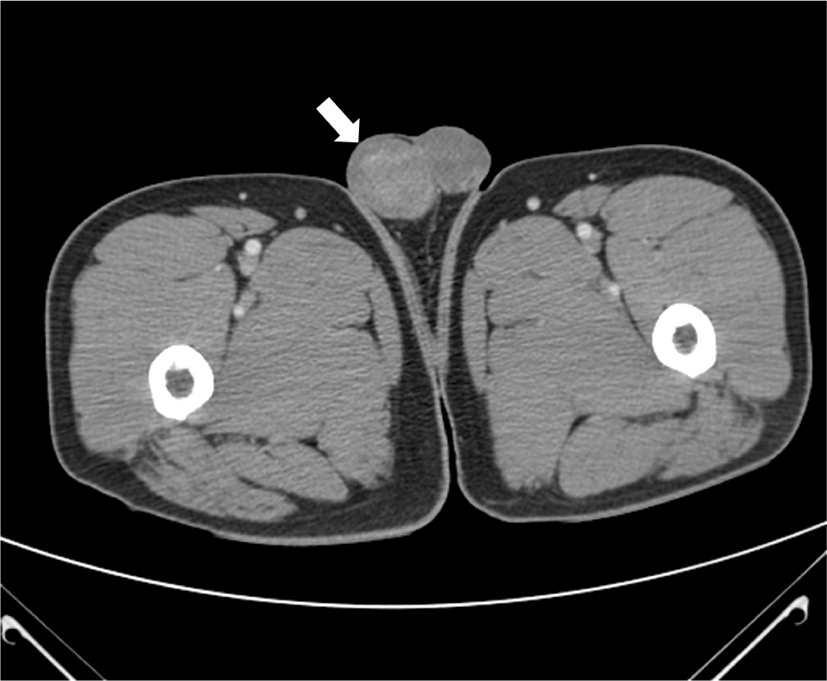
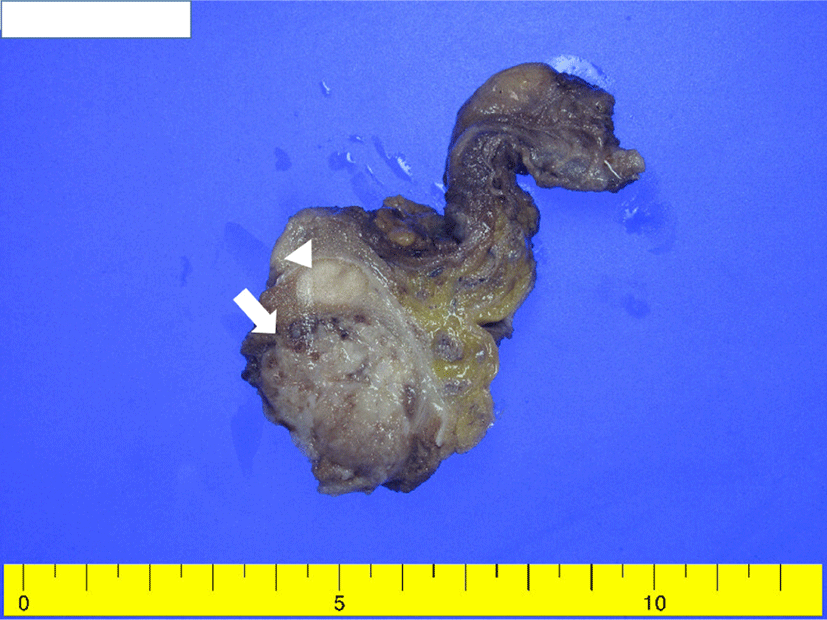
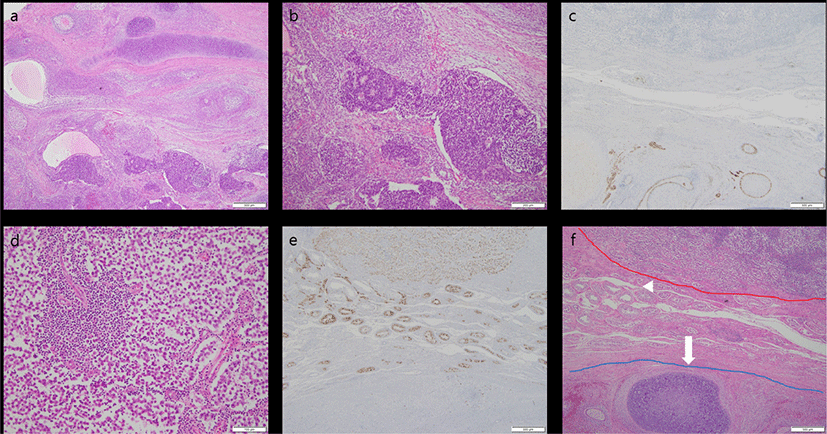
A 48-year-old, previously healthy man was referred for enlargement of the right scrotum over 2 months. Computed tomography revealed a well-defined, heterogeneous enhancing mass measuring 4 × 3 cm (Fig. 1). No remarkable findings were observed in other organs. Serum α-fetoprotein (AFP) level was increased (23.51 ng/mL) and beta-HCG level was unremarkable. Given a presumptive diagnosis of GCT, right orchiectomy was performed. Grossly, the lesion was composed of two separate well-circumscribed masses in the testicular parenchyma (Fig. 2). The larger mass underneath was heterogeneously brownish, soft, and lobulated, with mucinous and gelatinous materials. The smaller mass above was homogeneously creamy-white and solid. Microscopically, the larger tumor showed epithelial tissue, mesenchymal tissue, and immature neural tissue, which was composed of skin, respiratory tract tissue, intestinal tract tissue, bone, and cartilage (Fig. 3a). This larger tumor cells had cytological atypia and mitotic activity. The cartilage resembled a chondrosarcoma, and immature neuroectodermal tissue resembled neuroblastoma or medulloblastoma (Fig. 3b). In immunohistochemistry (IHC), the epithelial components were positive for pan-cytokeratin, mesenchymal components positive for vimentin, and immature neural tissue positive for CD56 and synaptophysin (Fig. 3c). This postpubertal-type teratoma was confined to testicular parenchyma without lymphovascular invasion and demonstrated testicular atrophy in the surrounding testis. In contrast, the smaller tumor was composed of pale cells showing diffuse and/or lobular configuration by fibrovascular septa containing lymphocytes (Fig. 3d). The tumor cells had distinct cell membranes and polygonal nuclei, and contained prominent nucleoli. IHC examination confirmed a diagnosis of a seminoma, wherein CD117 immunoreactivity demonstrated immature fetal type germ cells (Fig. 3e). There were obvious normal seminiferous tubules between the two GCTs (Fig. 3f). The patient’s postoperative course was uneventful, and he was discharged without any complications. No recurrence was observed during the two-year follow-up period.
DISCUSSION
A collision tumor is a rare tumor composed of two histologically distinct tumors coinciding; occurring in close proximity at the same anatomic site regardless of primary tumors and/or metastasis. Collision tumors have been described in many organs, including the stomach, uterine cervix, duodenum, urinary bladder, liver, lung, and ovary [2]. Previously, we reported the testicular collision tumor, comprising a seminoma and a metastatic cholangiocarcinoma [3]. The collision tumor that we have encountered this time is composed of two distinct primary GCTs (postpubertal-type teratoma and seminoma). This collision tumor need to be distinguished from two intermixed different histologic types, a mixed GCT.
About 95% of testicular neoplasms are of germ cell origin. A postpubertal-type teratoma is a malignant GCT composed of one or more germinal layers, while a seminoma is a malignant GCT resembling the primordial germ cells. Both are derived from a common precursor, germ cell neoplasia in situ (GCNIS). GCNIS shares common molecular features with seminomas [4] and is present in almost all seminomas. The ultrastructure of seminomas has shown evidence of epithelial differentiation [5] and increased DNA content, compared to nonseminomatous GCTs, suggesting that nonseminomatous GCTs evolve from seminomas as a consequence of gene loss [6]. Karyotypic analyses and loss of heterozygosity studies showed similar patterns in seminomas and nonseminomatous GCTs [7]. These imply that seminomas are also a precursor for nonseminomatous GCTs including postpubertal-type teratomas. Postpubertal-type teratomas usually occur as a component of mixed GCTs and are present in more than one-half of all mixed GCTs and in approximately 25% of all nonseminomatous GCTs [8].
If postpubertal-type teratomas and seminomas are found together within a mass, a mixed GCT can be suspected. There was no transition or histologic admixture at the interface. Both were spatially close, but completely distinct from each other pathologically, to be confirmed by the presence of normal seminiferous tubules between these two GCTs. There are some hypotheses to explain this collision tumor. GCNIS adjacent to seminomas or postpubertal-type teratomas can transform to another GCT. The distribution of GCNIS is characteristically multifocal and patchy, and adjacent profiles of seminiferous tubules may appear unremarkable with intact spermatogenesis. It is possible that two distinct GCNISs evolved to invasive GCTs at different rates. In fact, GCNIS has been identified in the residual seminiferous tubules of postpubertal patients with a testicular GCT. The postpubertal-type teratoma was larger than the seminoma. From this, it was presumed that the two GCNISs may have occurred at different times. Microenvironments such as inflammation and growth factors, aggravated by an earlier GCT may induce a later GCT. To a lesser extent, the occurrence could be coincidental. This patient should be managed like those with nonseminomatous GCTs, and not seminomas, because the prognosis is worse in those with nonseminomatous GCTs, compared to seminomas.
In conclusion, we described the first case of testicular collision tumor composed of two distinct GCTs. Given that these two GCTs originate from GCNIS, and that GCNIS is patchy distributed, there must have been more collision tumors including nonseminomatous GCTs that we overlooked. To prevent under-treatment, pathologist should perform close gross examination when GCNIS or seminomas are encountered. Awareness of this possibility might aid the diagnosis and management of testicular GCTs.
