INTRODUCTION
Colorectal cancer (CRC) is the third leading cancer worldwide [1]. The risk of colon carcinogenesis relates to chronic intestine diseases, such as inflammatory bowel disease, Crohn’s disease, and ulcerative colitis. Experimental evidence has suggested that rodent models of CRC have offered useful data on the occurrence and pathogenesis of colon carcinogenesis, involving cell transformation and the following events causing neoplastic lesion formation [2]. Also, previous researchers used the inflammation-induced CRC mice model to test the therapeutic efficacy of anti-tumorigenic substances [2]. Most CRC models include colon carcinogens, such as 1,2-dimethylhydrazine (DMH) and its metabolite, azoxymethane (AOM), because of their organotropic features [3]. The last tumorigenic metabolite of DMH induces DNA methylation in diverse organs, including the intestinal epithelial cells of colonic crypts, leading to cellular apoptosis with anti-proliferation and mutation in rodents and humans [4-6]. Notably, previous research demonstrated that AOM is a better CRC inducer than DMH because of greater stability and potency in dosing solutions [7]. Therefore, in most CRC mice models, we used dextran sodium sulfate (DSS) with AOM to investigate colon carcinogenesis development in DSS-induced colitis. In contrast, AOM initiates colon carcinogenesis, and DSS, a nongenotoxic carcinogen, promotes colon carcinogenesis by inducing colitis in rodents [8, 9].
Reactive oxygen species (ROS)-induced lipid peroxidation is a critical factor influencing oxidative stress of colonic epithelial cells [10]. Considering that cellular ROS accumulation causes DNA damage and genetic instability, lipid peroxidation in intestinal cells is a risk factor for colon carcinogenesis [10]. Additionally, several studies showed that antioxidant treatment and hypoxia adaptation for suppressing oxidative stress inhibited cancer carcinogenesis in animal models [11-14]. Therefore, the antioxidative substance and stress-based approach are promising therapeutic strategies for CRC treatment.
Pectin is a soluble dietary fiber formed from complex polysaccharides abundant in galactoside residues of various fruits [15]. It consists of four major polysaccharides: xylogalacturonan, homogalacturonan, rhamnogalacturonan I, and rhamnogalacturonan II [16]. Pectin oligosaccharides (POS) have attracted interest in exploring potential anti-tumorigenic candidates [16]. Furthermore, pectin has some chemopreventive effect on colon carcinogenesis [17]. Proper intake of pectin has been thought to be a protective agent against CRC because pectin has anti-carcinogenic and antioxidative properties and can reduce the transit time of feces in the intestine, which lowers the exposure of colonic mucosa to luminal carcinogens [18, 19].
Furthermore, butyrate, a metabolite of microbial fermentation of pectin, can induce apoptosis, inhibition of proliferation, or differentiation of tumor cells via ROS regulatory pathways [19-21]. Therefore, this study examined whether pectin serve as a chemopreventive agent by decreasing the formation of pre-neoplastic lesions on the colon in the AOM/DSS mice model. Also, we examined whether pectin decreases the level of lipid peroxidation end products, such as malondialdehyde (MDA) in feces, and inhibits the potential risk of cell injury by oxidative stress and lipid peroxidation.
MATERIALS AND METHODS
AOM and pectin was obtained from Sigma-Aldrich (St. Louis, MO, USA). DSS, whose molecular weight is 36,000–50,000, was obtained from MP Biomedical (Irvine, CA, USA).
Male institute for cancer research (ICR) mice (four weeks old) were obtained from Samtako Bio Korea (Osan, Korea) and housed in an isolating polycarbonate cage (five mice/cage). The temperature and relative humidity were set at 20 ± 2℃ and 50% ± 20%. Light and dark cycles were set at 12 hr each, and illumination intensity was maintained at 150–300 Lux. Additionally, AIN-76A purified diet was obtained from Central Laboratory Animal (Seoul, Korea). During the experiment, diets and litter were used after sterilization, and the animal experiment was conducted in compliance with the Guide for the Care and Use of Laboratory Animals of Chungbuk National University (CBNUA-1139-18-01; approval number from Chungbuk National University Institutional Animal Care and Use Committees).
After acclimation for one week, thirty mice (5 weeks old) were divided into two groups, including AOM/DSS (control) and 5% Pectin + AOM/DSS groups. Additionally, an AIN-76A-purified diet was supplied to the mice. The pectin group was given 50 mL of 5% Pectin in drinking water weekly. Then, AOM (10 mg/kg b.w) was intraperitoneally injected three times to the mice at 0, 1st, and 2nd weeks of the experiment to induce the formation of pre-neoplastic lesions in the colon. In the 3rd week of the experiment, distilled water containing 2% DSS was given for 7 d. The total experimental period is six weeks, and the detailed experiment schedule is described in Fig. 1.
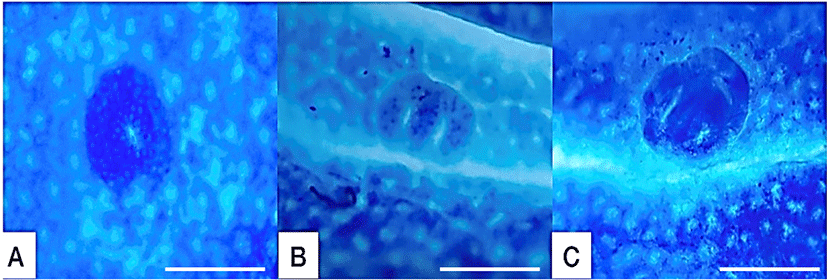
After colons were sampled, they were flushed with formalin, opened longitudinally, and fixed flat between filter papers in 10% neutral buffered formalin. Next, the formalin-fixed colon tissues were stained with 0.2% methylene blue solution. Finally, the entire number of ACF and AC in each focus were counted under a microscope (× 40 and × 100; Fig. 2).
Colon tissue was fixed in 10% neutral buffered formalin and paraffin-embedded, cut into multiple 4- μm sections, and stained with hematoxylin and eosin (H&E) for histopathological examination under a light microscope (× 100). In addition, sliced tissue samples were visualized using a light microscope (Olympus, Tokyo, Japan).
The dry feces were sampled from each cage for 1 d before sacrifice. First, samples were processed by adding 1 mL of distilled water to 0.3 g of dried feces. Then, they were incubated at 37℃ for 1 hr and thoroughly mixed during incubation. Next, they were then centrifuged for 15 min at 20,000×g, and the supernatant was collected and stored at −20℃ until use. MDA levels were measured using TBARS assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA). The MDA-TBA adduct formed by MDA and thiobarbituric acid (TBA) reaction under high temperature (90℃–100℃) and acidic condition was measured colorimetrically at 530–540 nm.
RESULTS
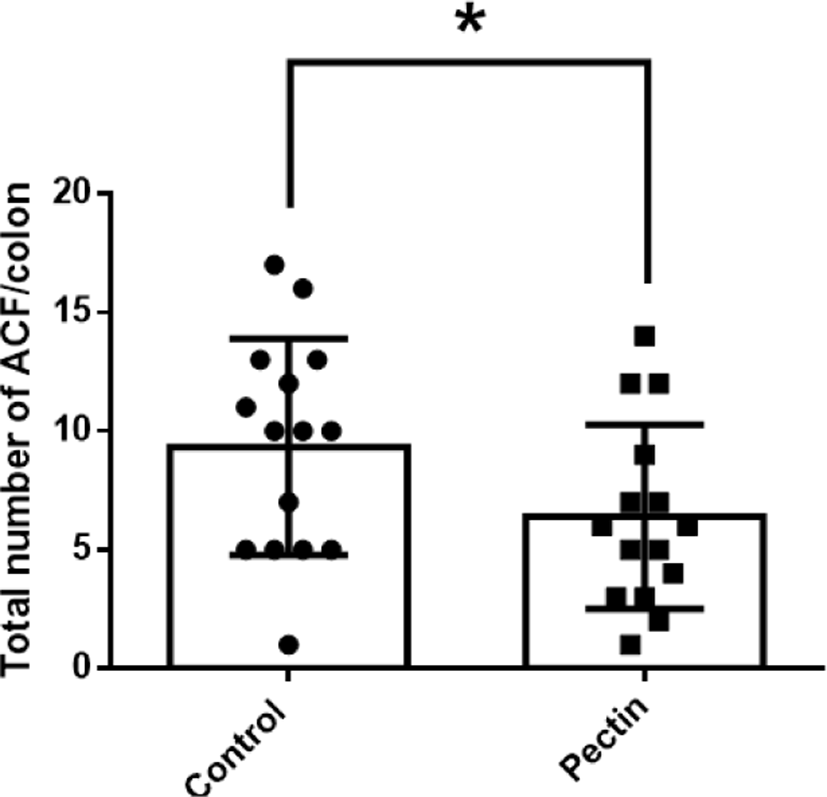
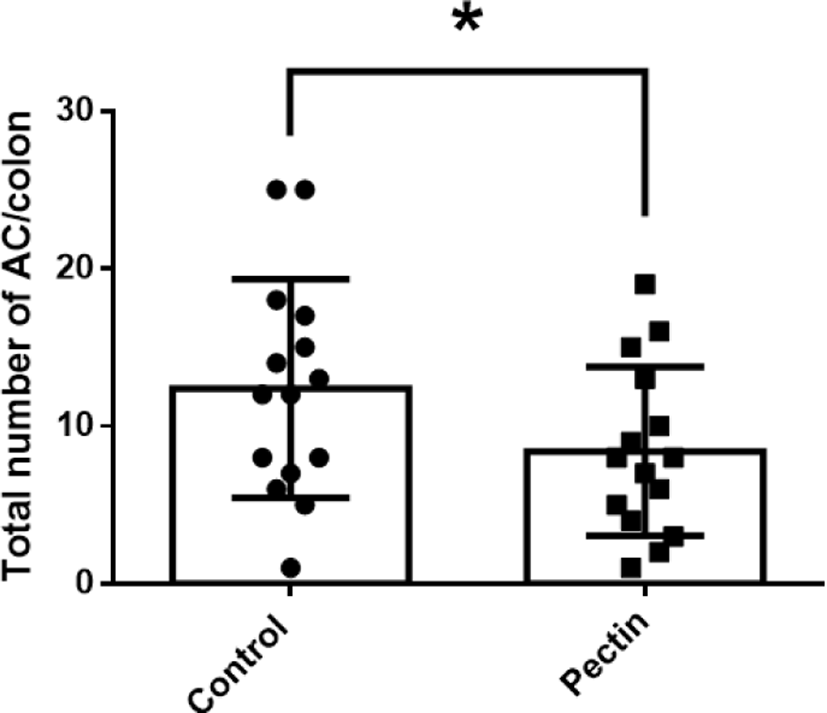
The body weights of mice in all experimental groups were measured weekly to investigate the effect of pectin supplementation on AOM/DSS mice. During the experiment, the body weights of mice in all experimental groups were increased for six weeks; the control group was slightly heavier than the pectin group, but there was no significant difference between them (Fig. 3). In addition, we found that pectin supplementation prevented AOM/DSS-induced hyperplastic changes and aberrant crypts formation (Figs. 4 and 5). ACF and AC formation are the earliest histological alterations in colon tissues during colon carcinogenesis. Therefore, we counted the total number of ACF and AC in colon samples of control and pectin groups to confirm the regulatory effect of pectin on AOM/DSS-induced colon carcinogenesis. As shown in Figs. 4 and 5, the total number of ACF in the 5% pectin group (6.4 ACF/colon) was significantly low compared with the control group (9.3 ACF/colon; p<0.05). Additionally, the total number of AC in the pectin group (8.4 AC/colon) was also lower than that in the control group (12.4 AC/colon; p<0.05). Thus, these findings indicate that pectin supplementation ameliorates AOM/DSS-induced hyperplastic change and AC formation in mice models.
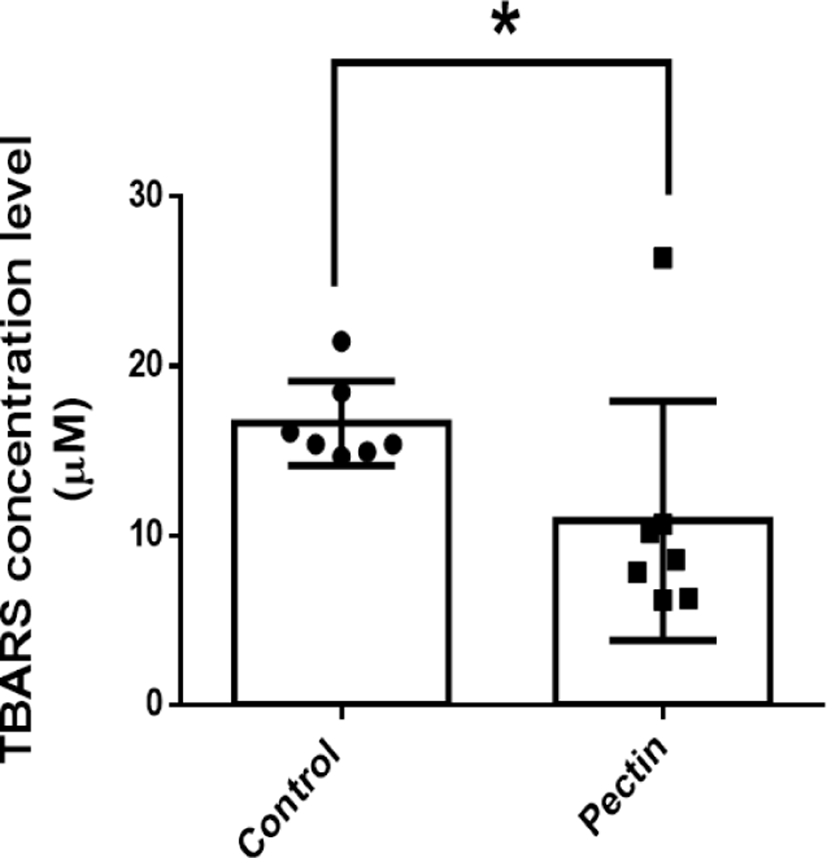
Fecal MDA concentration, as a marker of lipid peroxidation, is closely associated with oxidative stress of colonic epithelium. Therefore, to investigate the modulatory effect of pectin supplementation on oxidative stress in AOM/DSS mice, we performed TBARS assay with dry fecal samples. The TBARS concentration in the pectin group (10.4 μM) was lower than that in the control group (16.6 μM; p>0.05; Fig. 6). This finding indicates the antioxidative effect of pectin supplementation in AOM/DSS mice, suggesting the therapeutic potential of pectin in oxidative stress-related colon carcinogenesis.
DISCUSSION
This study showed that pectin supplementation inhibits AOM/DSS-induced colon carcinogenesis and lipid peroxidation in mice models. Recently, there have been many questions about whether fruits popularly consumed are beneficial to health or not. Therefore, this study was designed to determine whether pectin, the main component of some fruits, plays a protective role against colon carcinogenesis induced by AOM/DSS and cell injury by oxidative stress.
Our data showed that pectin supplementation ameliorated AOM/DSS-induced hyperplastic change in colonic tissues. Consistently, many previous studies showed that pectin is metabolized to butyrate by microbial fermentation in the intestine. This metabolite can induce apoptosis, inhibit proliferation, or differentiate tumor cells [20, 21]. POS inhibited oxidative stress and inflammation-activated signaling, suggesting a therapeutic candidate for colonic inflammation and related CRC incidence [16]. Additionally, the pectin matrix is a useful drug delivery system for colon cancer treatment [17]. Previous researchers have reported close relationships between intestinal inflammation and colon cancer [22, 23]. In the colitis model, it has been reported that the oral administration of POS inhibited DSS-induced disease activity index and weight loss [16]. However, this is inconsistent with previous findings; our study revealed that pectin supplementation did not affect body weight change. Furthermore, a previous mechanistic study demonstrated that the POS-inactivated MAPK signaling pathway suppresses the inflammation in CRC cells [24, 25]. Collectively, these findings indicate that POS and pectin reduce colonic intestinal inflammation in colitis-induced colon cancer.
Pectin, which acts as an antioxidant, can inhibit cellular damage induced by oxidative stress via unclear mechanisms. It may directly scavenge ROS, thus, avoiding contact with this toxic substance. Pectin may also act as a chelator of transition metals, such as iron and copper. Furthermore, pectin reacts with the superoxide radical, preventing its dismutation to hydrogen peroxide [26]. According to previous findings, POS normalizes the activity of redox system regulatory enzymes, such as glutathione reductase, glutathione peroxidase, and catalase [16, 27]. AOM-induced highly reactive electrophile induces cellular oxidative stress in colon tissue [28]. Since the inflammatory signaling pathway is closely linked to ROS regulatory machinery, the antioxidative and anti-inflammatory abilities of pectin are essential for pectin-ameliorated tumorigenesis of CRC [25].
Conclusively, the results showed that pectin decreases the number of pre-neoplastic lesions and formation of lipid oxidation induced by AOM/DSS, i.e., pectin can inhibit the development of colon carcinogenesis in the AOM/DSS mice model. Thus, this study supports that proper intake from fruits or supplements prevents colitis-associated colon carcinogenesis.
