Introduction
Q fever, a zoonosis caused by C. burnetii, occurs worldwide. The incidence of human Q fever was 0.33 (number of case/100,000 persons) in Korea, 2019. Those of other countries including Australia, Spain, France and United States were 2.3 in 2018, 0.7 in 2017, 0.3 in 2017, and 0.04 in 2018 [1–3], respectively. Cattle, sheep, and goats and other species (algae, arthropods, and ticks) are usually the potential source of human infection. Goats have been linked to human outbreaks of the recent Q fever, particularly in Europe and the United States [4, 5]. Human may be infected through direct contact (e.g., by ingestion of contaminated animal products or skin inoculation), but the primary mode of transmission is to inhale dust contaminated with C. burnetii [6].
The incubation period of human Q fever is typically around 20 days, with a range of 3 to 30 days [7]. As many as 50% of human Q fever cases are asymptomatic; symptomatic infections most commonly present as a non-specific febrile illness that may occur in conjunction with pneumonia or hepatitis [8]. Acute Q fever is characterized by sudden onset of fever to 40°C, chills or sweats, severe headache with retro-orbital pain, weakness, nausea, vomiting, diarrhea, non-productive cough, and abdominal or chest pain. Untreated, the fever can persist for up to 9 to 14 days [7]. Approximately 30% to 50% of patients develop pneumonia. Pregnant women may be at risk for abortion, stillbirth, pre-term delivery, or low infant birth weight and developing chronic Q fever [9–11]. In animals, infection caused by C. burnetii is also asymptomatic. The signs related with chronic Q fever in goats, sheep, and cattle are infertility, abortion, and the birth of full-term dead or weak, lower-weight offspring. Abortions typically occur over a 2- to 4-week period and may affect 5 to 50% of the flock. Most of the abortions occur in the last trimester of pregnancy or near term [12].
Previous serologic and bacteriologic studies have shown that C. burnetii is widely distributed among host animals in Korea [13, 14]. In cattle, the seroprevalence is 9.5%–11.6%, and in goats is 15%–19% [14–16]. In healthy people, the seroprevalence is 1.5% and 10.2% in slaughterhouse workers [17].
Since 2015, the number of Q fever patients in Korea has been sharply increasing, and since 2014, the number of goat farms has been also continuously increasing [18]. This increase in the size of goat breeding was likely to affect the increase in Q fever patients. Recently, goat raising by urban to rural returners has been increasing. In 2018, 11,961 households immigrated into countryside for the agro life, of which 373 (3.1%) were engaged in raising livestock including 58 (15.5%) goat farming [19]. Urban to rural returners tend to choose goats that are relatively easy to breed, but have little knowledge on goat’s diseases. In particular, the unsanitary handling of the fetuses, placentas from aborted goats, and weak neonates can be a serious problem in public health because it might contain infectious agents. Therefore, this report aims at making prepare a detailed standard control and provide regular educations on biosecurity of Q fever for beginner farmers.
Cases
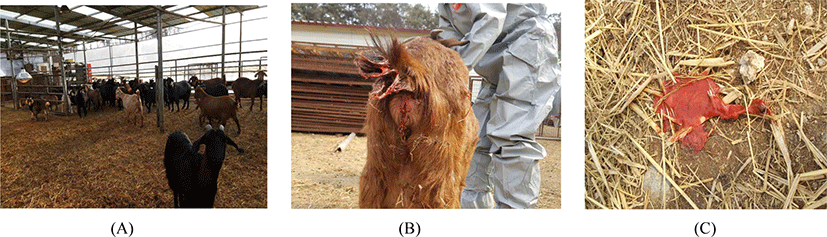
Since January 2018, continuous abortions and pre-term delivery occurred in a goat farm located in Cheongju, Chungcheongbuk-do, Korea. This farm raised 77 goats (22 males, 55 females) in a general housing condition. The soil ground of the barn was covered with rice straw litter. Four walls of the barn were rapped with vinyl, and the roof was made of iron panel and transparent plastic plate (Fig. 1A).
According to history, since the early January, sporadic abortions and premature deliveries (3–4 month-old fetuses) had occurred for about 40 days from 6 pregnant goats. And other goats gave birth to weak offsprings. In the beginning, the farmer considered the sporadic abortions as an adverse effect of foot and mouth disease vaccination done in late December 2017 and sent the specimens to Chungcheongbuk-do Institute of Veterinary Service and Research for the investigation on the cause of the abortion.
We conducted gene amplifications to detect the agents possible to cause abortion in caprine, which included the followings; Brucella abortus, Coxiella burnetii, Campylobacter fetus, Listeria monocytogenes, Leptospira spp., Chlamydophila abortus, Neospora caninum, Toxoplasma gondii, bovine viral diarrhea virus (BVDV), infectious bovine rhinotrachitis virus (IBRV), Ainovirus (AINOV), Chuzan virus (CHUV), Akabane virus (AKAV), Ibaraki virus (IBAV), bovine ephemeral fever virus (BEFV). DNA/RNA was extracted from various tissue samples (liver, brain, kidney, spleen and heart) using a Viral Gene-spinTM Viral DNA/RNA extraction kit (Intron, seongnam, Korea) according to the manufacturer’s instructions. The primer sequences used for the PCR are shown in Table 1. For amplification of the viral genes, commercial viral detection kits were used for AINOV, CHUV, AKAV, IBAV and BEFV (MEDIAN DiagnosticsTM, Chuncheon, Korea) and for BVDV (PowerCheckTM, Kogenebiotech, Seoul, Korea). For Q fever, C. burnetii specific gene (is1111) was identified from the different samples of the fetus (spleen, liver, lung, brain and amniotic fluid) and seropositivity were examined by enzyme-linked immunosorbent assay (IDEXX Laboratories, Westbrook, ME, USA) from the serum all aborted cases.
| Target organisms | Target gene | Sequence (5’→ 3’) | Product size (bp) | References | |
|---|---|---|---|---|---|
| Bacteria | Brucella abortus | 16s RNA |
TCGAGCGCCCGCAAGGGG AACCATAGTGTCTCCACTAA |
905 | [20] |
| Coxiella burnetii | is1111 |
TATGTATCCACCGTAGCCAGTC CCCAACAACACCTCCTTATTC |
687 | [21] | |
| Listeria monocytogenes | Hly |
CGGAGGTTCCGCAAAAGATG CCTCCAGAGTGATCGATGTT |
234 | [22] | |
| Leptospira spp. | secY |
CTGAATCGCTGTATAAAAGT GGAAAACAAATGGTCGGAAG |
285 | [23] | |
| Chlamydophila abortus | Pmp |
ATGAAACATCCAGTCTACTGG TTGTGTAGTAATATTATCAAA |
300 | [24] | |
| Protozoa | Neospora caninum | Nc-5 |
CTCGCCAGTCAACCTACGTCTTCT CCCAGTGCGTCCAATCCTGTAAC |
350 | [25] |
| Toxoplasma gondii | B1 |
GGAACTGCATCCGTTCATGA CAGACGAATCACGGAACTG |
501 | [26] | |
| Virus | IBRV | gC |
TACGACTCGTTCGCGCTCTC GGTACGTCTCCAAGCTGCCC |
476 | [27] |
For further diagnosis against all 77 goats in the farm, the positivity of antigen and antibody of C. burnetii were confirmed from 54 (70.1%) and 63 (81.8%) goats, respectively (Table 2). Furthermore, extensive contamination of C. burnetii was identified from specimens of 12 different environmental places (4 barns, 3 playgrounds, 2 male-only-barns, 2 outside of the barn, 1 maternity barn). We even experienced that the aborted fetuses were shedding sanguineous vaginal discharge on the different place of the floor (Fig. 1B, 1C).
| Sex | Conventional PCR | Serology by ELISA | |||
|---|---|---|---|---|---|
| No. of the tested | Positive cases | (%) | Positive cases | (%) | |
| Female | 55 | 43 | (78.1) | 42 | (76.3) |
| Male | 22 | 11 | (50.0) | 21 | (95.4) |
| Total | 77 | 54 | (70.1) | 63 | (81.8) |
Since 2016 the farmer and his wife as urban to rural returners had begun to raise goats for a living without any notions and/or knowledges on the public health problems derived from animal diseases. Although consecutive abortions had occurred since January of 2018, they could not have dealt it with any biosecurity treatments. Around 6 days after disposing aborted fetuses, the farmer had suffered from high fever (39°C), chills and severe pain in joints and muscles. Farmer’s wife also got sick with same symptoms in February 2018. However, they did not go to the hospital because they considered it as common cold. On the suggestion of the veterinary officials who diagnosed goat Q fever, they received a Q fever test by indirect immunofluorescence assay (IFA, IgG/IgM kit [Focus Diagnostics, Cypress, CA, USA]) and hospitalized at Chungbuk National University Hospital as soon as high level of C. burnetii antibodies titer were confirmed (Table 3). Moreover, another human C. burnetii infections were also confirmed from 7 of 14 veterinary officials who had visited to the farm for protective measures and additional 2 laboratory technicians who had prepared blood samples for ELISA. Even though all officials visited the farm wore personal protective equipments (goggle, mask, gloves, hoody cover all and boots covers) in the farm, high titer of C. burnetii antibodies were detected by IFA (Table 3). Especially, 5 of 9 IFA positives showed severe Q fever symptoms as the farmer had experienced.
Discussion
Q fever occurs worldwide because C. burnetii has wide host range and high environmental resistance. In Korea, Q fever in human was designated as a class 4 notifiable disease in 2006 and has been managed as a class 3 infectious disease since 2020. From 2006 to 2014, around 10 cases were reported every year. Yearly reported cases increased sharply from 27 to 177 in 2015 and 2019, respectively [28]. It is insisted that these increase might be related to the increase of goat rearing [29]. Unlike cattle, the number of goat has increased more than double from 2014 to 2018 [18]. In our field experiences, when compared to the past situations, the occurrence of goat Q fever tends to increase gradually, and as shown in this report, massive goat Q fever infections have been frequently occurring in recent years. However, there are rare studies about epidemiological relationship between goat and human in Korea.
The incubation period in human is typically about 20 days with a range of 3 to 30 days. About 50% of the cases are asymptomatic [7]. In this case, the symptoms from the farmer and other officials appeared acute and severe. This means that a large amount of C. burnetii contaminated in the barn was introduced into the patients.
The epidemiology of C. burnetii suggested that it is transmitted to persons principally through the inhalation of dried aerosol particles and contact with infected animals and their reproductive tissues [6]. It is known that C. burnetii can be present in placenta from normally delivered goats. In addition to abortions, normally delivered infected goats contribute to the environmental contamination, which should therefore be considered as a major risk factor [30, 31]. The source of infection to the farmer and the veterinary officials might be postpartum secretions (amniotic fluid, vaginal discharge) and dispersed dusts containing the agents from the soil or feces on the floor of the barn. Since there was no proper operational space in the farm, the infected goats and weakly born neonates were not separated from the healthy herd until the veterinary officials took proper biosecurity actions. Aborted fetuses and postpartum secretions actually led to extensive environmental contamination to the farm. Protective measures on severely contaminated farm can be very hazardous to veterinary officials even they wore personal protective equipment. Especially, in cruel working condition such as restraining heavy goats under the hot weather, wearing face mask and goggles might not be effective for protecting the infection. To make safe, prophylactic measures including vaccination or antibiotic treatment on goat herd can be more suitable to control C. burnetii infection.
On epidemiological inspection on the introduction of agent to this farm, the carrier was the male goat which have been to another goat farm for breeding. Diseases causing abortion were not observed in this farm over the last couple of years. The male goat was the only susceptible animal moved outside in October and return to the farm in November 2018. C. burnetii specific gene (is1111) were also identified from the blood sample of the male goat. Generally, in order to prevent incest breeding, many goat farms in Korea borrow and lend male goats to/from other farms without any quarantine procedure. Some studies reported that transmission of C. burnetii from male to female animal or human via sexual contact was possible [32, 33]. We were presumed that introduction of Q fever into this farm was accomplished by the infected male goat contacted sexually with female goats in this farm. Male-goat-borrowing or lending for breeding could also contributes Q fever spreading. However, since C. burnetti is known to be frequently detected in ticks, the vector-borne infection is possible to goats [34].
Currently, the public health concerns are rising due to the increasing occurrence of Q fever in Korea, and various surveys and researches have been attempted to analyze the sources of human Q fever and to suggest control policy for animal Q fever. Above all, the biggest thing to reduce the incidence of human Q fever is to control animal Q fever, especially on goat farm. According to a survey on perception of Q fever among livestock workers in Korea, 80.2% of livestock farmers perceived bovine brucellosis. However, only 3.1% of them were aware of Q fever [35]. This high percentage of perception on brucellosis was due to the quarantine policies on bovine brucellosis that have been implemented for many years in Korea. On the other hand on Q fever, the preventive policies is very insufficient, and it is necessary to prepare a detailed standard control policy including treatment on infected individuals, vaccination on herd, epidemiological investigation and considerable guide line for stamping out consideration at the farm level and to provide regular hygiene and quarantine education for a beginner farmer on farming hygiene.
