Introduction
Dopamine (DA), an important catecholaminergic neurotransmitter, is involved in controlling diverse brain functions, including motor control, emotion, cognition, and reward behaviors [1–3]. DA neurons are located in the substantia nigra (SN) and ventral tegmental area (VTA). They send the major axonal projection and comprise the nigrostriatal pathway which is interconnected with the striatum and thought to control voluntary movements [4].
Dopaminergic receptors are divided into two subclasses: D1-like (including D1 and D5 receptors) and D2-like (including D2, D3, and D4 receptors) [5, 6]. Many dopamine receptor agonists have been developed to replace levodopa with an additional goal to provide more continuous dopaminergic stimulation [7]. Rotigotine, a DA D2-like receptor agonist, has been developed for treating Parkinson’s disease (PD) as a transdermal patch. It has high affinity for both D2 and D3 receptors [8, 9]. Preclinical studies have shown that rotigotine can act as a D2-like receptor agonist [10]. Rotigotine can act as an agonist for all dopamine receptors with a moderate selectivity for D2-like subtypes, particularly D3 receptor [7].
Rotigotine has been reported to have neuroprotective effects against glutamate toxicity [11]. It has also been found that rotigotine has neuroprotective effects against toxicity of 6-OHDA or other neurotoxins [12, 13]. However, it is necessary to investigate whether rotigotine acts through DA D2 or D3 receptor and influences DAergic neurons in vivo and in vitro. Thus, the objective of this study was to investigate whether rotigotine could exhibit neuroprotective effect through DA D2 or D3 receptors against 6-OHDA induced toxicity.
Materials and Methods
Groups of ICR mice (8 weeks old; Samtaco bio Korea, Osan, Korea) were kept in specific pathogen free conditions in an adjusted room with 12/12 h light and dark cycles. Vaginal plugs of female mice housed with a male mouse for 10–12 h were confirmed at 8:00 AM. The day of plug discovery was designated as embryonic day 0 (E0). Embryos were removed after deep inhalation anesthesia by the mother of mouse. Three mice were used for each group regardless of sex (male or female). Animal experimental protocols were approved by the Animal Ethics Committee of Gyeongsang National University (Approval Number: GNU-151217-M0070).
Mouse primary cultures were performed as described previously [14] with minor modifications. Briefly, developmental brains of mice on E12 were resected in Hanks balanced salt solution. Neurosphere cells were isolated from microglial cells, oligodendrocytes, and debris via density gradient. In a 0.1 mg/mL poly-L-lysine-coated well, isolated neurosphere cells were seeded at a density of 100,000 cells per cm2 in a culture medium containing Neurobasal A, B27 supplement, basic fibroblast growth factor, L-glutamic acid, and gentamicin. These cells were subcultured at 37°C in a humidified atmosphere containing 5% CO2 for 7 days. To investigate the effect of rotigotine on DAergic neurons, 6-OHDA and rotigotine hydrochloride (Tocris Bioscience, Bristol, UK) were administrated into the primary culture. Samples for Western blot were prepared at 72 h after treatments.
Mice were anesthetized with xylazine (5 mg/kg) and zoletile (10 mg/kg) and located on a stereotaxic frame. To estimate neuroprotective effects of rotigotine, drugs (6-OHDA 0.4 mg/kg; rotigotine 3 μg/kg, 15 μg/kg) were unilaterally injected into the SN of mice midbrains with coordinates of 3.0 mm posterior to bregma, 1.4 mm lateral to the midline, and 4.0 mm ventral to the skull surface based on Mouse Brain in Stereotactic Coordinates [15]. These injections were performed using a Hamilton syringe. The syringe was maintained in place for 5 min after the injection. Skin was sutured and mice were returned to the cage for anesthesia recovery.
At seven days after injecting rotigotine with 6-OHDA in the SN for tyrosine hydroxylase (TH) expression, mice were deeply anesthetized with xylazine and zoletile and sacrificed by cervical dislocation. Brain samples were post-fixed for 48 h and embedded in paraffin wax. Samples were serially sectioned at a thickness of 5 μm with a rotary microtome for immunohistochemistry. Nonspecific reactions were blocked with 3% fetal bovine serum in phosphate buffered saline (PBS) at room temperature for 1 h. Slides were incubated with mouse monoclonal primary TH antibody (TH; diluted 1:1,000; Millipore, Temecula, CA, USA) at room temperature for 1 h. Samples were then washed 3 times with PBS and incubated with anti-mouse IgG-Horseradish peroxidase (HRP; 1:1,000, Bio-Rad, Hercules, CA, USA) for anti-TH antibody. After washing, samples were reacted with 3,3-diaminobenzidine tetrahydrochloride (DAB, Vector laboratories, Burlingame, CA, USA) for anti-mouse IgG-HRP. When color was developed, slides were mounted with permount (Fisher Scientific, Hampton, NH, USA).
To estimate expression levels of TH, mouse primary culture was administrated with 6-OHDA (1 μM) and treated with rotigotine and antagonists L-741,626 (Tocris Bioscience, Bristol, UK) or GR 103691 (Tocris Bioscience, Bristol, UK) for 72 h. Cultured cells were washed with PBS and total cell lysates samples were prepared using a cell lysis buffer (1 M Tris-HCl [pH 8.0], 5 M NaCl, 1% NaN3, 10% SDS, 10% NP-40, and 0.5% C24H39NaO4). An equal amount of sample was loaded onto a 10% polyacrylamide gel. After electrophoresis, proteins were transferred to polyvinylidene membranes. Nonspecific reactions were blocked with 5% bovine serum albumin in 0.1% Tween 20 for 1 h. Membranes were then incubated with anti-TH (1:1,000; Millipore, Temecula, CA, USA) and anti-actin (1:10,000; Millipore, Temecula, CA, USA) at 4°C overnight. After washing 5 times with 0.1% tris-buffered saline with Tween 20 (TBST), membranes were incubated with HRP conjugated secondary antibodies at room temperature for 1 h. Proteins were detected using an enhanced chemiluminescence reagent (Antigen, Seoul, Korea). Densitometry was carried out using Image J.
Data are presented as mean ± S.D. of at least three independent experiments. Analysis of variance (ANOVA) was performed to compare multiple groups based on average values and grasp correlations through hypothesis verification. p-values less than 0.05 were considered statistically significant.
Results
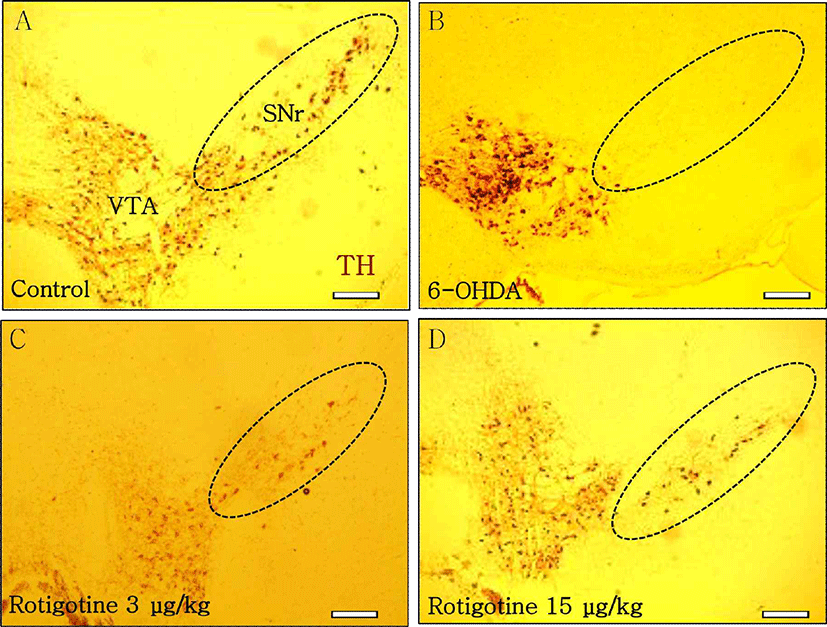
Intracranial injection of 6-OHDA can induce partial degeneration of DAergic neurons in SN of the mouse midbrain, mimicking the pathology of Parkinson’s disease model in vivo [16]. To investigate the effect of DA D2-like receptor agonist on DAergic neurons, rotigotine was administrated in the SN. Immunohistochemistry was carried out to identify the localization of TH positive cells. In the control group, TH positive cells were observed in VTA and SN (Fig. 1A). Unilateral injection of 6-OHDA (0.4 mg/kg) caused significant loss of TH positive cells in the SN, although TH positive cells were still observed in the VTA (Fig. 1B). DA D2-like receptor agonist rotigotine (3 μg/kg, 15 μg/kg) inhibited the loss of DAergic neurons in the SN of 6-OHDA-induced mouse model of PD at 7 days after administration (Figs. 1C, 1D).
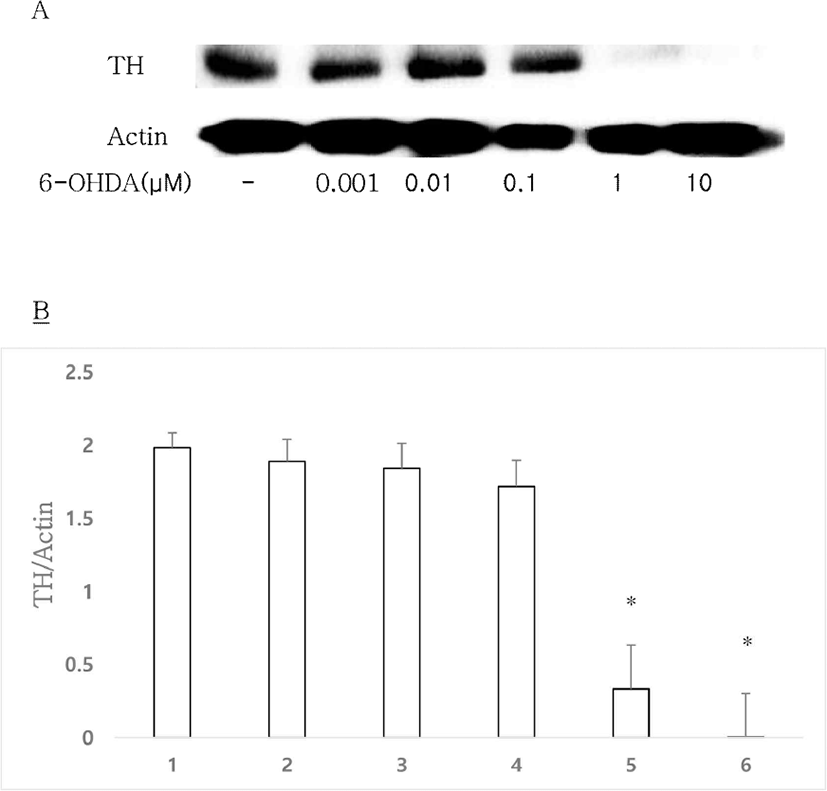
6-OHDA, a selective DAergic neurons neurotoxin, can be used to establish an experimental model of DAergic neuron degeneration [16]. To investigate the effect of 6-OHDA in vitro, 6-OHDA was administrated to mouse primary culture. The control group showed clear TH expression, whereas expression levels of TH were significantly decreased by 6-OHDA at 1 and 10 μM (Figs. 2A, 2B). Therefore, 1 μM 6-OHDA was used at a basal concentration in the following in vitro experiments.
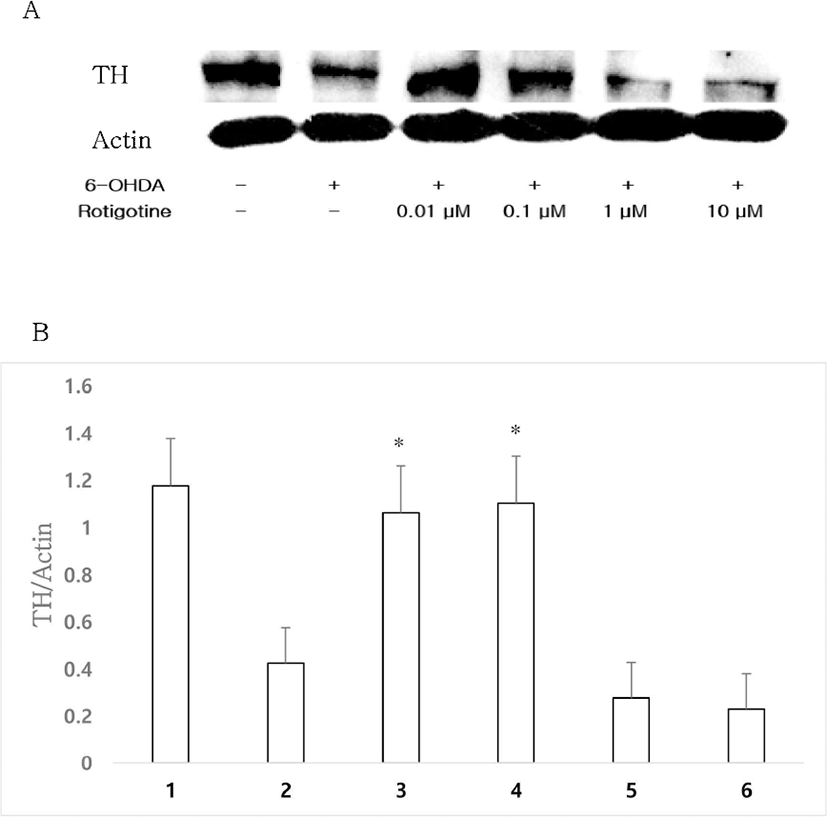
To investigate the optimal dose of DA D2-like receptor agonist rotigotine known to exhibit neuroprotective effects, rotigotine and 1 μM 6-OHDA were administrated to a mouse primary culture. Treatment with 6-OHDA (1 μM) resulted in a significant loss of TH expression, whereas treatment with rotigotine (0.01, 0.1 μM) inhibited the loss of TH expression to control level (Figs. 3A, 3B). In the following experiments to investigate whether rotigotine could strongly act through D2 or D3 receptors, rotigotine was used at a basal concentration of 0.01 μM.
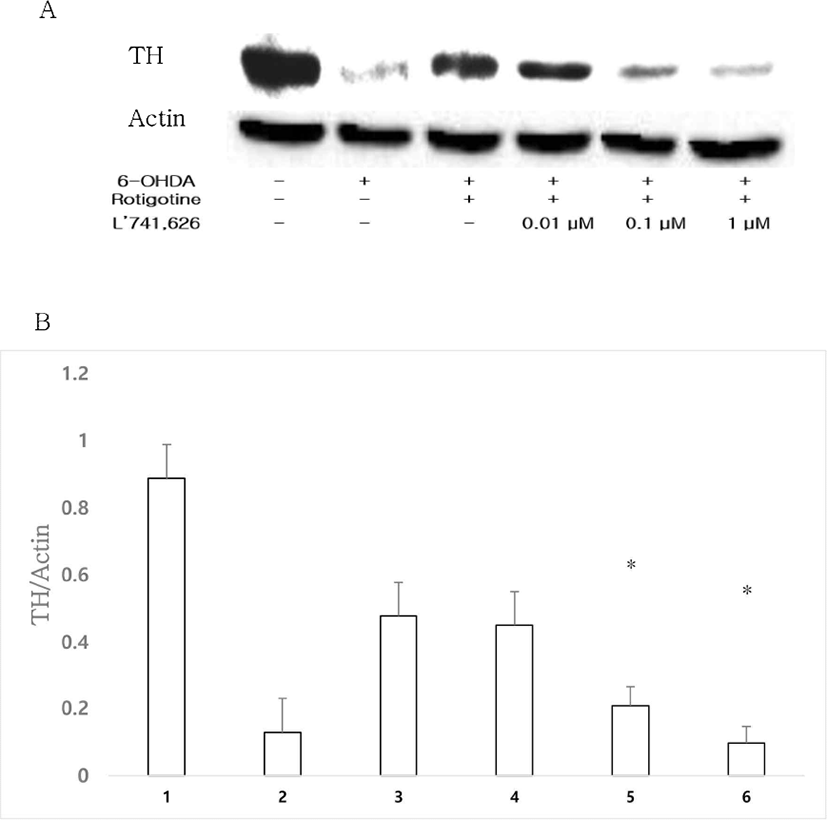
DA D2 receptor selective antagonist L’741,626 (0.01, 0.1, 1 μM) was administrated along with 0.01 μM rotigotine and 1 μM 6-OHDA to investigate whether rotigotine would act through D2 or D3 receptors. After treatment with L’741,626 and rotigotine, TH expression was decreased in a dose dependent manner compared to that in the rotigotine group (Figs. 4A, 4B).
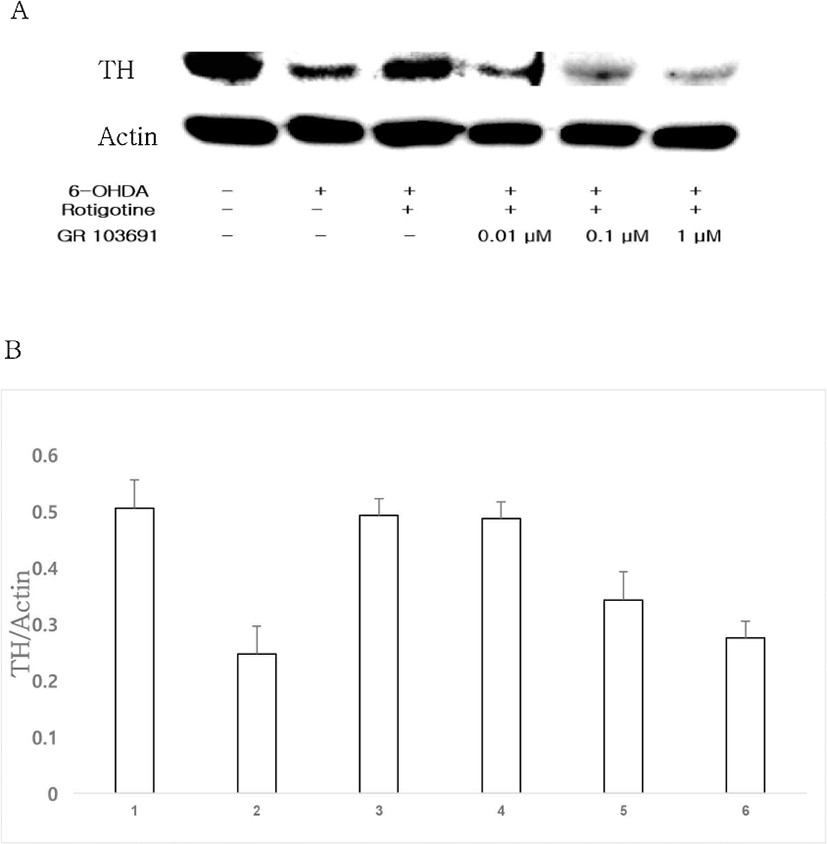
To compare between D2 and D3 receptors, D3 receptor selective antagonist GR 103691 (0.01, 0.1, 1 μM) was administrated with 0.01 μM rotigotine and 1 μM 6-OHDA. Compared to TH expression in the rotigotine group, TH expression in the group treated with GR 103691 (0.01, 0.1, 1 μM) and rotigotine was decreased in a dose-dependent manner (Figs. 5A, 5B). When results of groups treated with D2 and D3 receptor antagonists were compared, it was found that rotigotine acted more strongly through D2 receptor than through D3 receptor.
Discussion
DA is synthesized from tyrosine, an essential amino acid. TH can converts tyrosine to DA precursor form L-DOPA. Dopamine depletion is one of the most important features of PD, a common movement disorder [17]. Intracranial 6-OHDA injection is an established method for chronic DA depletion [18]. This compound is absorbed through plasma membrane DA receptors, after which it is rapidly oxidized, resulting in production of reactive oxygen species (ROS) and semiquinone that can induce DAergic neurons and selective cell death [19]. Treatment with L-DOPA can dramatically alleviate DA degeneration movement dysfunction. However, its continuous use can induce other movement dysfunctions such as dyskinesia. Using DA agonists is a promising DA degeneration disorder therapy that can reduce dosage of L-DOPA and delay the onset of its side effects [20, 21].
There are two distinct types of DA receptors: D1-like receptor and D2-like receptor, the latter of which includes D2, D3, and D4 receptors. These receptors are classified based on the existence of G protein-coupled structure [22]. Many dopamine receptor agonists can protect DA neurons against 6-OHDA [16, 23, 24]. Although 6-OHDA-induced mouse model is a well-known PD model, effects of DA D2-like receptor agonist in mice with 6-OHDA-induced lesions are not completely understood yet. In this study, we produced mice with 6-OHDA-induced lesions and measured the loss of DAergic neurons following administration of rotigotine, a dopamine D2-like receptor agonist. Rotigotine was developed for the treatment of PD. It has high affinities for D2 and D3 [8, 25]. Many studies have reported that rotigotine has strong affinities for D2 and D3 receptors [7, 10]. In a previous in vitro study, rotigotine has shown neuroprotective effects [11]. However, whether rotigotine might exhibit stronger neuroprotective effect through D2 or D3 receptor remains unclear. Results of the present study revealed that treatment with rotigotine increased the expression of TH in 6-OHDA-induced neurotoxicity. The present study also showed that the expression of TH through DA D2 receptor was increased more than that through DA D3 receptor. These results suggest that DA D2-like receptor agonist rotigotine exhibits neuroprotective effects on DAergic neurons and that it acts more strongly through DA D2 receptor than through DA D3 receptor.
