Introduction
Granulomatous meningoencephalitis (GME) is a non-suppurative inflammatory central nervous system (CNS) disease in dogs [1]. Although the etiology is not clearly elucidated, it is immunologically classified as a T-cell mediated autoimmune disease [1, 2]. Because of similar neurological and morphological features on magnetic resonance imaging (MRI), GME is difficult to distinguish from solid brain tumors in clinical practice [1, 3–5]. GME is categorized as an immune-mediated CNS inflammatory disease referred to as meningoencephalitis of unknown etiology (MUE). Histopathologic examination of affected brain tissues is essential to obtain a definitive diagnosis. However, ante-mortem brain samples are challenging to collect. Thus, a tentative diagnosis of GME is made based on MRI and cerebrospinal fluid (CSF) analysis findings. After a tentative diagnosis is obtained, combination therapy with conventional immunosuppressive agents plus glucocorticoids is used as a standard therapy similar to other immune-mediated disorders [1–3, 6].
Imatinib mesylate is a selective tyrosine kinase inhibitor (TKI) that competitively blocks the ATP binding site of tyrosine kinases (TKs). It has a potent therapeutic effect on cancer by blocking the phosphorylation TKs, which are abnormally activated in malignant process [7]. In human medicine, imatinib mesylate has been used not only to control abnormal tumor growth but also to treat different autoimmune diseases. It is effective for the management of multiple sclerosis (MS) in humans similar to non-suppurative immune-mediated inflammatory brain diseases in dogs [7]. Furthermore, one canine study has shown that imatinib mesylate can be a treatment option for MUE in dogs [8].
Herein, we describe a case of MUE in a dog that was successfully managed with imatinib mesylate. Furthermore, to increase our knowledge on the use of imatinib mesylate in dogs with MUE, this study aimed to provide a detailed information on serial changes in clinical and MRI findings.
Case Description
A 4-year-old, female, Maltese dog with bilateral hind limb ataxia was brought to Gyeongsang National University Animal Medical Center (GAMC). The patient initially presented with neurological sign approximately 5 months before presentation to GAMC. Five months back, MRI was performed at a local animal hospital. Results showed a uniformly, ill-defined ovoid mass-like lesion in the temporo-occipital region (Fig. 1). The lesion was hyperintense on T2-weighted (T2W) images and isointense on T1-weighted (T1W) and fluid-attenuated inversion recovery (FLAIR) images. Moreover, an intense and uniform enhancement was observed on contrast-enhanced T1W (CET1W) images; vasogenic edema that was hyperintense on T2W images was identified around the lesion. The appearance of the lesion was consistent with an intra-axial brain mass, and the patient was presumptively diagnosed with a primary brain tumor at a local animal hospital. Then, treatment with oral hydroxyurea (50 mg/kg every 48 h; Hydrine capsule; Korea United Pharm., Seoul, Korea) and prednisolone (0.5 mg/kg every 12 h; Solondo tablet; Yunhanmedica, Cheongwon, Korea) was initiated. The clinical signs improved gradually. However, ataxia in the hind limbs recurred while tapering the dosage of prednisolone 5 months after the initial treatment. The dosage of prednisolone was again increased to the last effective dose. However, ataxia only slightly resolved. Hence, the patient was referred to our hospital.
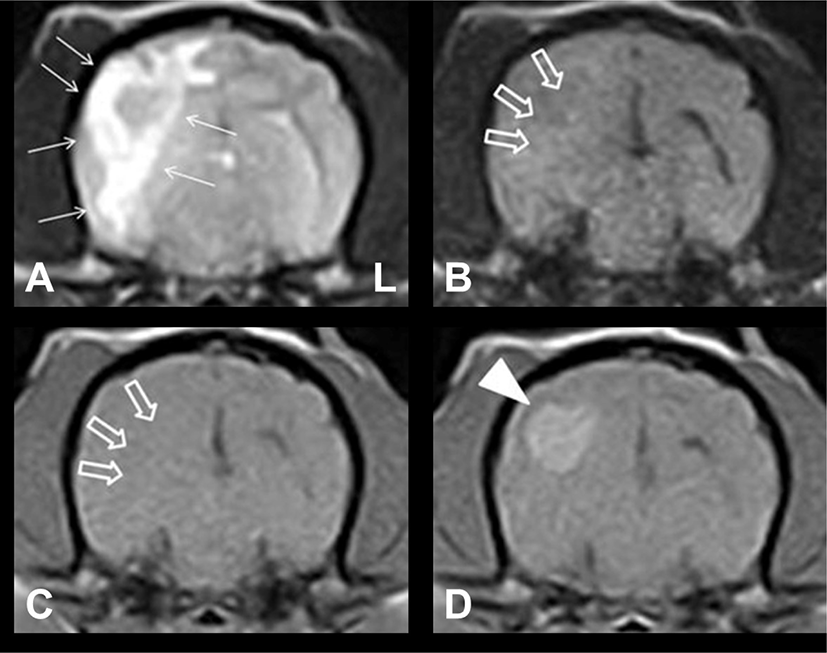
Upon admission, neurological examination revealed hind limb ataxia, and conscious proprioception was reduced in both hind limbs. No other neurological abnormalities were identified. To re-evaluate the intracranial lesions, we performed brain MRI using a 0.4T scanner (APERTO; Hitachi Medical Corporation, Tokyo, Japan) and CSF analysis (obtained from the atlanto-occipital cistern tap using a 22-gauge needle). An ill-defined lesion with vasogenic edema and mass effect was observed in the right temporo-occipital region (Fig. 2). These findings were significantly different from those on MRI performed 5 months back. The lesion was hypointense on T1W images, iso- to hyperintense on T2W and FLAIR images, and irregularly enhanced on CET1W images. These MRI findings indicated that the lesion was more indicative of an inflammatory brain disease than a primary brain tumor. Conventional CSF analysis revealed an increased nucleated cell count at 5 cells/uL (reference range: <5). Moreover, the protein concentration was 15 mg/dL (reference range: <25). The slightly elevated levels of nucleated cell count in the CSF seemed to be attributed to the prednisolone previous administration. Cytological examination revealed mononuclear cell pleocytosis. Based on the response to prednisolone therapy, irregular inflammatory changes in the lesion on MRI, and CSF analysis results, the patient was diagnosed with MUE. Furthermore, the morphologic features of the lesion on MRI were consistent with focal GME in the brain.
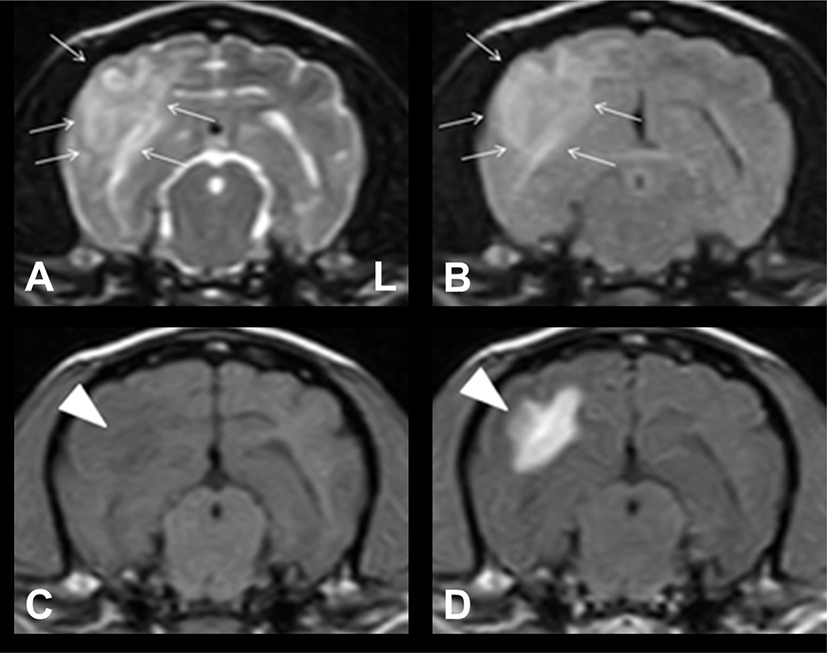
We added oral imatinib mesylate (10 mg/kg every 24 h; Glivec; Novartis, Stein, Switzerland), and the dosage of prednisolone was increased to 1 mg/kg twice daily. Hydroxyurea was discontinued. A rapid improvement in neurological signs was observed after the initiation of imatinib mesylate treatment. To assess for treatment response, MRI was performed again at the same level, as shown in Fig. 2, 71 days after the initiation of imatinib mesylate treatment. The inflammatory lesion with vasogenic edema observed on the previous MRI had almost resolved, and concomitant midline shift had also completely resolved (Fig. 3).
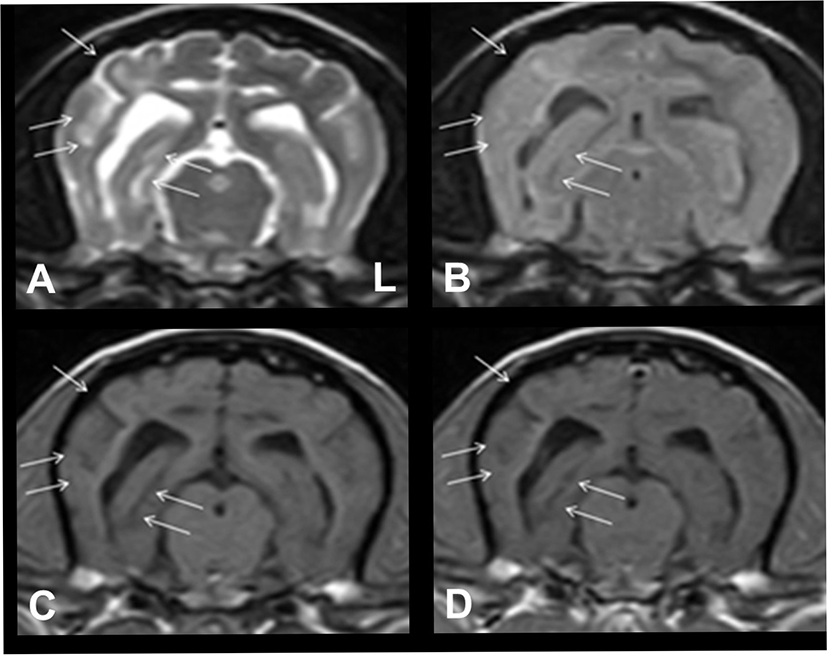
The patient continuously received treatment with imatinib mesylate at the same dosage. The dosage of prednisolone was gradually tapered to 0.3 mg/kg once daily. The patient did not experience any relapse of neurological signs. Then, 171 days after the initial presentation, the patient showed mild partial seizure-suspected episodes in the left hind limb. Thus, the dosage of prednisolone was increased to 0.5 mg/kg twice daily, and oral zonisamide (5 mg/kg every 12 h) was added to the treatment regimen. Thereafter, the patient did not present with any neurological signs. However, 259 days after starting imatinib mesylate therapy, the patient suddenly died from acute pancreatitis despite supportive therapy.
Discussion
GME is a canine inflammatory brain disease, which was originally classified as an intracranial tumor due to its morphological features [1, 2]. However, to date, a single granuloma-like lesion, as in the case of focal GME, is extremely difficult to distinguish from some solitary solid brain tumors, such as histiocytosis, primary CNS lymphosarcoma, and meningioma [2, 9]. Histopathologic examination via ante-mortem brain tissue biopsy or postmortem necropsy is essential to obtain a definitive diagnosis of GME. Diagnosis is usually achieved via CSF analysis and MRI examination in clinical practice [1]. In the current case, the initial MRI scan conducted at a local hospital revealed an ovoid mass-like lesion with homogeneous contrast enhancement. Back then, the mass was considered a primary brain tumor. However, the appearance of the lesion on the second MRI conducted at GAMC had changed to an ill-defined inflammatory lesion with irregular contrast enhancement. The MRI features and CSF results were consistent with GME of the prosencephalon in canines. Furthermore, the morphological changes in the lesion on serial MRI after a period of treatment were consistent with inflammatory brain changes. In addition, the patient responded well to dose titration (prednisolone) before the initial presentation and to the combined treatment with prednisolone and imatinib mesylate. Accordingly, the possibility of GME is more strongly considered than that of primary brain tumor. Thus, the patient was tentatively diagnosed with GME [9].
TKs are key mediator enzymes in normal cell signaling that strictly regulate normal cell growth and cell differentiation [7]. They function as an on/off switch in several cellular functions and are abnormally activated in various malignancies and inflammatory diseases. Targeted therapy for dysregulated TKs has been considered a promising strategy for the treatment of some malignancies in human and veterinary practices [10]. Furthermore, the efficacy of inhibiting dysregulated TKs in some immune-mediated diseases has been suggested [11]. Multiple sclerosis (MS) is an immune-mediated inflammatory disease in humans and is characterized by immune-mediated damage in the normal myelin and axons of the CNS. TKs play a key role in this immune-related process of MS, and clinical trials have assessed the efficacy of blocking TKs in MS treatment [12]. Recently, MS was considered neuropathologically similar to GME, which is considered a T-cell-related autoimmune CNS disorder, in dogs [1, 13, 14].
There are several strategies used for targeting TKs and TKIs, which include the use of a class of drugs that inhibit one or more TKs. Imatinib mesylate is a multi-targeted inhibitor of platelet-derived growth factor, c-Kit, c-Abl, c-Fms, Lck, Flt-3, and MAPK [11]. Imatinib mesylate was first introduced as an antineoplastic agent for treating leukemias in humans. However, a few human studies have shown the clinical efficacy of imatinib mesylate in rheumatoid arthritis, Crohn’s disease, and MS as it blocks the activity of imatinib mesylate-targeted TKs [12, 15]. Recently, two veterinary studies have shown the abnormal expression of imatinib mesylate-targeted TKs in canine GME and necrotizing encephalitis (NE) tissues, and one of these studies showed the treatment efficacy of imatinib mesylate in patients with GME [8, 13]. Their results included the overexpression of platelet-derived growth factor receptor, vascular endothelial growth factor receptor, c-Kit, and c-Abl in the affected brain tissues.
The current study showed the treatment efficacy of imatinib mesylate in GME. After initiating imatinib mesylate treatment, there was a significant improvement in the lesion in the right temporo-occipital lobe and related perilesional edema and mass effect on the adjacent structures on serial MRI (Figs. 2 and 3). Furthermore, the patient had been doing well after imatinib mesylate treatment. Moreover, there were no evident neurological signs, other than minor partial seizure-suspected activity, during the treatment period. Hence, the relevant effect of imatinib mesylate was confirmed in canine GME.
The focal type of GME has a relatively long-term survival compared with the disseminated form. However, GME generally has a poor prognosis, ranging from weeks to months [6]. The survival time of the patient was 259 days after therapy with imatinib mesylate (410 days after the initiation of prednisolone therapy alone). Although imatinib mesylate treatment was delayed due to the antitumor therapy associated with the misdiagnosis of brain tumor, the patient significantly responded to imatinib mesylate treatment. Furthermore, the patient suddenly died from acute pancreatitis, not from neurological etiologies. Therefore, if early imatinib mesylate treatment was initiated and if acute pancreatitis was properly managed, the patient would have survived significantly longer than 259 days.
Long-term immunomodulating therapy with corticosteroids and adjuvant immunosuppressive drugs in dogs with MUE can cause various adverse effects (gastrointestinal upsets, sporadic infection, etc.) [16, 17]. In terms of adverse effects (e.g., superficial edema, muscle cramps, and nausea), imatinib mesylate is a relatively safe drug for veterinary patients compared with human patients [18]. However, long-term treatment with imatinib mesylate can also affect the normal organs of veterinary patients with active cell cycle and can exert adverse effects, including gastrointestinal problems, hepatotoxicity, and bone marrow suppression [19]. In the current case, we did not find any adverse effects associated with imatinib mesylate during the treatment period. Thus, we surmised that imatinib mesylate might be a very safe and effective treatment modality to canine patient. The patient was severely affected by acute pancreatitis approximately 8 months after the initiation of imatinib mesylate therapy. Long-term corticosteroid therapy was previously considered a risk factor for canine pancreatitis. However, the mechanism has not been fully elucidated to date. Furthermore, recent studies have not supported this concept, and they recommend that clinicians should assess for other risk factors associated with pancreatitis in patients receiving corticosteroid therapy [20]. Therefore, the long-term use of prednisolone in this patient might not be correlated with acute pancreatitis, and other factors had affected the development of the condition. Although imatinib mesylate has no adverse effects on the pancreas based on human and veterinary literature, further studies should be conducted to investigate whether imatinib mesylate has any adverse effects on the pancreas.
Herein, we report the use of imatinib mesylate in a dog with GME. The patient’s response indicated that combined therapy with imatinib mesylate plus prednisolone can be well tolerated and can be a good alternative to conventional MUE treatment. The major limitation of this case report is that only one patient was assessed, and there were no available data on immunohistochemistry (IHC) results, including information on the related-TKs of the affected brain tissues of the patient. Therefore, further long-term, controlled, IHC-based studies with a larger population of dogs with MUE must be conducted to establish a new treatment protocol for the use of imatinib mesylate. Furthermore, there are only few data on the potential effects of imatinib mesylate in other autoimmune diseases in the veterinary field. However, the use of this drug for the treatment of immune-mediated diseases might be extremely promising based on the results of the current study.
