Introduction
Canine inflammatory mammary carcinoma (CIMC) similar to human inflammatory breast cancer (IBC) is a very aggressive, metastatic type of cancer with a grave prognosis [1–4]. What makes CIMC so aggressive is its angiogenic and angioinvasive nature, which contributes to its metastatic potential. The histopathological hallmark of CIMC is evidence of neoplastic emboli in dilated lymphatic vessels [1]. More specifically, similar to IBC, CIMC has increased vascular density, a high rate of endothelial cell proliferation with high levels of angiogenic and lymphangiogenic factors [3]. The mechanism behind this type of tumor angiogenesis is not well understood; however, previous studies have introduced a new type of tumor angiogenesis called vasculogenic mimicry that may play an important role in the progression of inflammatory mammary cancer [5, 6]. Vasculogenic mimicry is a phenomenon describing in de novo creation of microvascular channels by deregulated endothelial-like tumor cells [5–8]. Thus, instead of creating new vascular channels from pre-existing blood vessels, tumor cells transform into endothelial-like cells and function as a channel for blood cells. The formation of vasculogenic mimicy is evident not only in inflammatory mammary caners but also in other malignant carcinomas and sarcomas of dogs and human beings [9–14]. However, it is still speculative if the vascular channels become real blood or lymphatic vessels lined with endothelial cells, and then interconnected to the preexisting vessels resulting in distant tumor metastasis.
Epithelial-mesenchymal transition (EMT) has been proposed to be a plausible mechanism by which neoplastic cells form vasculogenic mimicry in tumors [15, 16]. EMT in which TWIST-1 plays significant roles is a crucial for cancer progression and metastasis, providing tumor cells the ability to transform and migrate to other regions [17–21]. EMT may be involved in vasculogenic mimicry formation by utilization of different cellular markers such as VCAM-1 [7, 21, 22]. Vascular endothelial growth factors were highly immunoreactive in CIMC [7, 22].
In this study, we tried to investigate the development process of vasculogenic mimicry by neoplastic cells in CIMC based on histopathological and immunohistochemical analyses.
The CIMC was from a 14-year-old female Shih-tzu. Grossly, it was somewhat movable dark-reddish with adhesion to adjacent tissue, sized 6.2 cm in diameter, and bloody dark turbid exudate was released from the tumor.
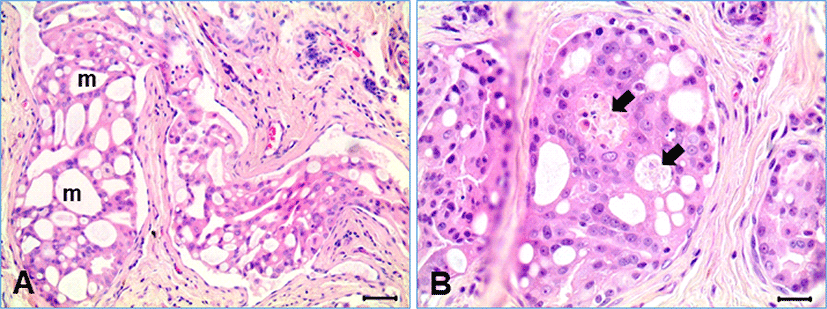
Histologically, the mammary tumor had typical features of CIMC, characterized by commonly found tubular solid tumor emboli within the lymphatic vessels surrounded by desmoplastic fibrous connective tissue (Fig. 1A). Most of the tumor masses were present within the lymphatic vessels lined by simple squamous endothelial cells. Invading neoplastic cell masses, not related to the lymphatic vessel, were sometimes found, but very rare. The neoplastic cells within the lymphatic vessels were forming variable sizes of round-shaped channels. Degenerative and necrotic cell groups were multifocally found in the solid neoplastic cell mass inside the tumor emboli, resulting in formation of vasculogenic mimicry (Fig. 1B).
Immunohistochemical stains were performed using the appropriate antibodies for pan-cytokeratin, vimentin, VCAM-1, MECA-32, TWIST-1, and Ki-67 (Table 1). The neoplastic cells represented strong immunoreactivity for pancytokeratin and weak to mild expression for vimenin (Fig. 2A and 2B). The endothelial cells forming the lymphatic vessels containing tumor emboli were negative for pancytokeratin, but strong positive for vimentin. In the hyperplastic mammary glands and ducts, the glandular epithelia indicated strong positive for pancytokeratin immunostain, but negative for vimentin, while the spindle cells (myoepithelial cells) surrounding the hyperplastic glands were strong positive for both pancytokeratin and vimentin. The neoplastic cells of CIMC expressed immunoreactivity for VCAM-1, although its expression was weaker than the vascular endothelial cells (Fig. 2C). The surface of the neoplastic cells forming vasculogenic mimicry within the intravascular tumor masses were relatively intense for VCAM-1 immunostain. Meanwhile, the endothelial cells containing embolic neoplastic cell masses indicated strong expression for VCAM-1. The neoplastic cells of CIMC indicated cytoplasmic expression for MECA-32 (anti-panendothelial cell Ag) (Fig. 2D). Immunohistochemistry for TWIST-1, a significant protein for EMT, represented nuclear positivity in many of the neoplastic cells of CIMC (Fig. 2E). The frequency of positive cells was different depending on location; positivity was highest as 100% in the neoplastic cells forming the vasculogenic mimicry within the neoplastic cell masses, while some negative cells were evident in the neoplastic cells in solid pattern. The endothelial cells lining the lymphatic vessels containing tumor emboli and the endothelial cells of small vessels in connective tissue were heterogenous. In the hyperplastic glands, some cells represented positive for TWIST-1, but the spindle cells surrounding the mammary glands were negative. The Ki-67 labeling index of the CIMC neoplastic cells was low as indicating under 10%, irrespective of the location of neoplastic cells (Fig. 2F).
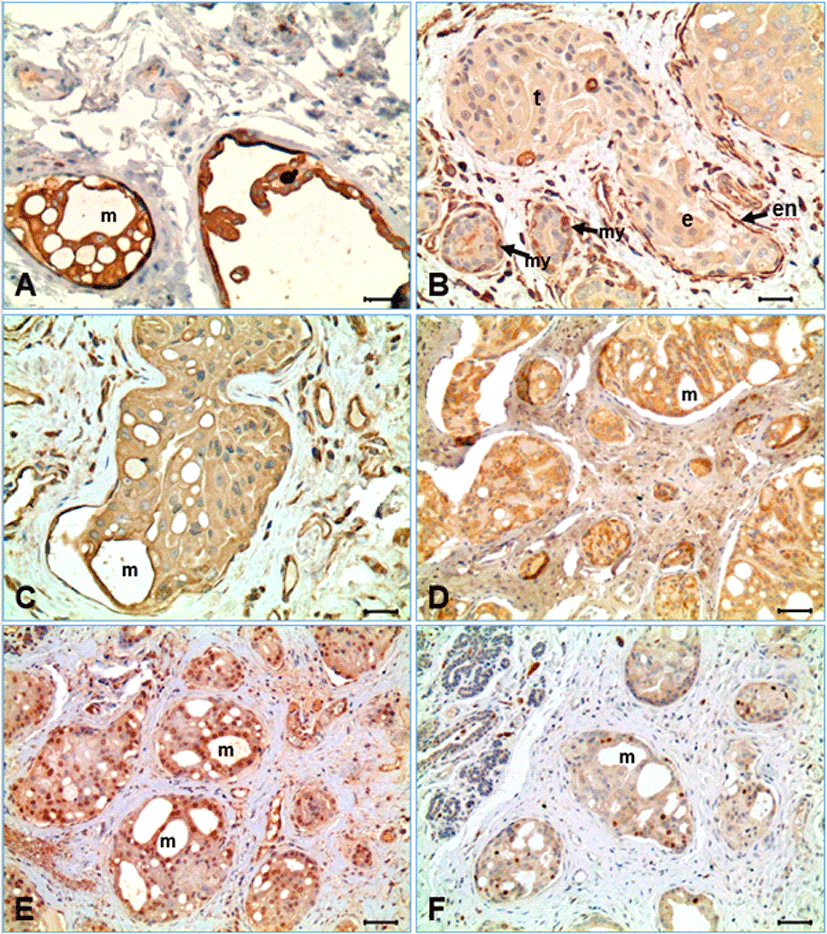
In our study, most of the neoplastic cells (>95%) were present in the form of emboli in lymphatic vessels with squamous endothelial cells, which is well documented characteristics of CIMC as well as in IBC in humans [1–6, 10, 21–23]. However, it is still not understood why lymphatic tumor embolism is so common in the mammary cancer and if the newly formed lymphatic vessels are originated from preexisting lymphatic vessels or formed by neoplastic cells. Because CIMC is well known to have high angiogenic behavior, researchers have studied different mechanisms that can possibly explain this concept. Vasculogenic mimicry is one of many theories researched that may contribute to explaining CIMC’s aggressive nature [7, 18, 23]. Vasculogenic mimicry is a phenomenon describing in de novo creation of microvascular channels by deregulated endothelial-like tumor cells [6–8, 23]. In our study, the embolic tumor masses were characterized by formation of variable sizes of small channels, as shown in Fig. 1. The neoplastic cells were often transformed into flat cells like endothelial cells, surrounding a vessel-like structure. It was a kind of epithelial mesenchymal transition in morphology. The formation of small channels in the tumor embolic masses seemed to be initiated from cell degeneration ad necrosis. Those flat neoplastic cells were strongly positive for cytokeratin, indicating they have still the epithelial characteristics. The neoplastic cells also expressed vimentin protein, which is an intermediate filament present in the mesenchymal cells, although the expression was relatively weak compared with that of endothelial cells and fibroblasts (Fig. 2). Acquisition of the vimentin protein by neoplastic cells may be related to the process of mesenchymal transition, as also suggested by the previous studies [16, 21, 24].
VCAM-1 and MECA-32 antibodies were chosen to observe the presence of angiogenesis and lymphangiogenesis in neoplastic cells because of its significant role in creating new vessels. As results, the neoplastic cells were shown to have immunoreactivity for VCAM-1 and MECA-32, a pan-endothelial cell antigen, suggesting the possibility that the neoplastic cells could transform into endothelial cells of vessels by EMT. This is further supported by serial morphological changes identified by histological and other immunohistochemical investigations. Strong association with EMT in the vasculogenic mimicry formation process was suggested in the present study by the expression pattern of TWIST-1 that plays a critical role in EMT mechanism; TWIST-1 was strongly expressed, particularly in the neoplastic cells surrounding the vasculogenic mimicry in the tumor emboli. The possible role of TWIST-1 regulated EMT for vasculogenic mimicry formation was supported by a strong positive correlation between TWIST-1 expression and vasculogenic mimicry formation [21]. According to our results, we suggest the process of forming lymphatic vessels by neoplastic cells in CIMC as followings. Neoplastic cells first form a circular ring creating a channel similar to the structure of a blood vessel or lymphatic vessel. Then, the neoplastic cells undertake epithelial mesenchymal transition, as the round neoplastic cells morphologically transform into flat endothelial-like cells. This structural change has been noted and researched by observing EMT in other neoplastic cells [5, 6]. After careful analysis of VCAM-1 expression, these channels then appear to relocate and ultimately embed into surrounding connective tissue outside of the vessels. Ki-67 was also used to determine the rate of tumor proliferation in CIMC. Even though CIMC showed high capacity of neoplastic cells, Ki-67 expression was low in our study. This may indicate why malignancy of CIMC highly depends on vasculogenesis and metastasis to other regions rather than cell proliferation.
Our study may provide a possible angiogenic mechanism behind CIMC, but further research is greatly required to fully comprehend CIMC’s vasculogenic and aggressive behavior. Further studies supporting this type of angiogenic mechanism may pave a way for developing new therapeutic strategies.
