Introduction
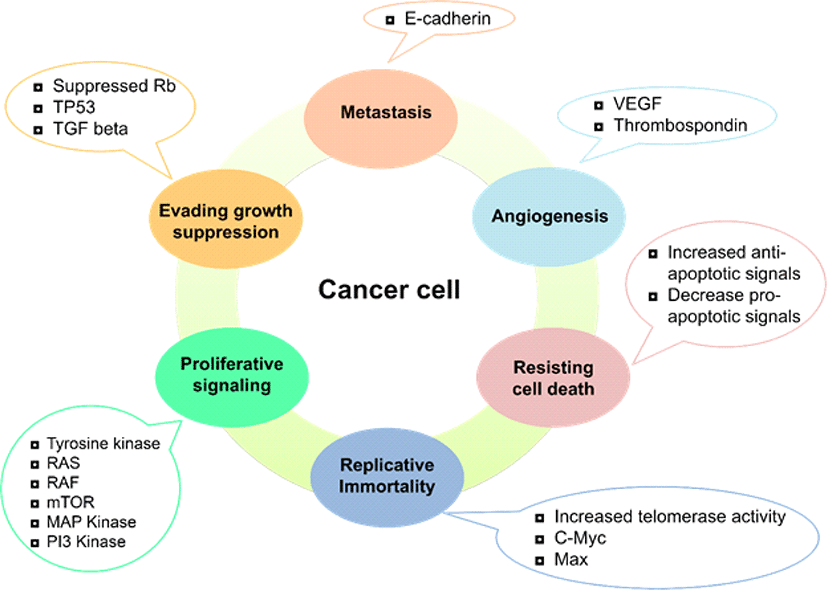
Cancer is the second leading cause of death in humans after heart-related disorders in cancer, there is an imbalance between cell division and cell death. Uncontrolled division of healthy cells triggered by several factors is the leading cause of different types of cancer [1]. Depending on the affected body part, cancer can be divided into five classes that include leukemia, lymphomas, sarcoma, carcinoma, and central nervous system-related cancer. Cancer has a high incidence, quick progression of the disease, a long period of latency and reduced prognosis rate. There are many factors involved in the development of cancer, for instance, geographical area, race, and age. Internal causes may trigger cancer, such as genetic mutation, impaired apoptotic function, and hypoxia. External causes that contribute to cancer may be prolonged exposure to ultraviolet rays, radiation, stress, smoking, and pollution [2]. Conventional cancer treatments include chemotherapy, radiotherapy, and immunotherapy, where chemotherapy is of significant concern for weak tumor tissue accessibility. The demand for high doses and the non-selective nature of the therapeutic options available are associated with a range of adverse drug reactions [3]. Six characteristics contribute to tumorigenesis and differentiate cancer cells from normal healthy ones Fig. 1 [2, 4, 5].
Increasing the proportion of plants in the diet has been a dietary target for reducing the risk of chronic diseases. Plants can provide excellent sources of nutrients that contribute to health [6]. Flavonoids are the most popular groups of plant secondary metabolites that occur widely throughout the entire plant kingdom. They are a large group of low molecular weight substances traditionally considered to be derivatives of phenylchromones. Flavonoids were subsequently known, using a broader definition, as compounds characterized by a carbon skeleton C6-C3-C6 in which the components C6 were aromatic rings and C3 a heterocyclic [7].
Flavonoids can be classified into flavonols (such as quercetin, kaempferol, isoquercetin, etc., found in onions, apples, berries, kale, leeks, broccoli, blueberries, red wine, and tea), flavones (such as glycosides of luteolin, chrysin, and apigenin, commonly found in fruit skins, parsley, and celery), isoflavones (such as genistein, daidzein and glycitein present in leguminous plants, mainly soy and soy products), flavanones (such as naringenin, eriodictyol and hesperidin exclusive of citrus fruits), flavanols (such as epicatechin, catechin, gallocatechin, epigallocatechin, epigallocatechin gallate and also polymeric forms or condensed tannins as found in cocoa and tea), and anthocyanidins (such as pelargonidin, cyanidin, and malvidin, found in red wine and berry fruits. Flavonoids have been recognized for antioxidant properties since more than 40 years ago. However, the biological activities of flavonoid go beyond antioxidant properties, [8]. Several studies of human and animal cervical cancer cells have shown that polyphenols and their derivatives have the potential for antioxidants and anti-cancer [9, 10].
Programmed cell death (PCD) is a cell suicide mechanism which is mediated by an intracellular program. PCD balances cell death and survival when equilibrium is broken and it activates multiple signal pathways [11]. PCD is the sequence of events leading to the organized and controlled destruction of cells [12]. PCD plays a crucial role in animal growth and homeostasis of the tissues. It is now well known that abnormal regulation of this process is associated with a wide variety of human diseases, including cancer [13]. The cell death mechanism serves two primary functions; 1) as an antagonist in the mitotic cell division holding excessive cell elimination regulated; 2) by discarding mutated cells as a defense mechanism in diseases such as cancer. In addition to inhibiting cell proliferation, the induction of cell death through several forms of PCD is now becoming a major strategic solution to cancer prevention and treatment [14].
The term apoptosis derived from the Greek terms apo, for “apart” and ptosis, for “fallen”, which means shedding of leaves. All multicellular organisms have a strictly regulated pathway of cell growth, maintenance and removal mechanisms are necessary for cell number control, based on a balance between cell proliferation and cell elimination [15]. Apoptosis features a series of well-defined morphological changes. Initially, the volume of cells decreases or shrinks, a pyknotic nucleus is formed, and the chromatin is condensed along the nuclear membrane. It follows the failure of nuclear power and the development of small cellular fragments or apoptotic bodies. The last shift is the phagocytosis of intact apoptotic bodies, which prevents the release of cellular contents into the surrounding areas and blocks any inflammatory response [15].
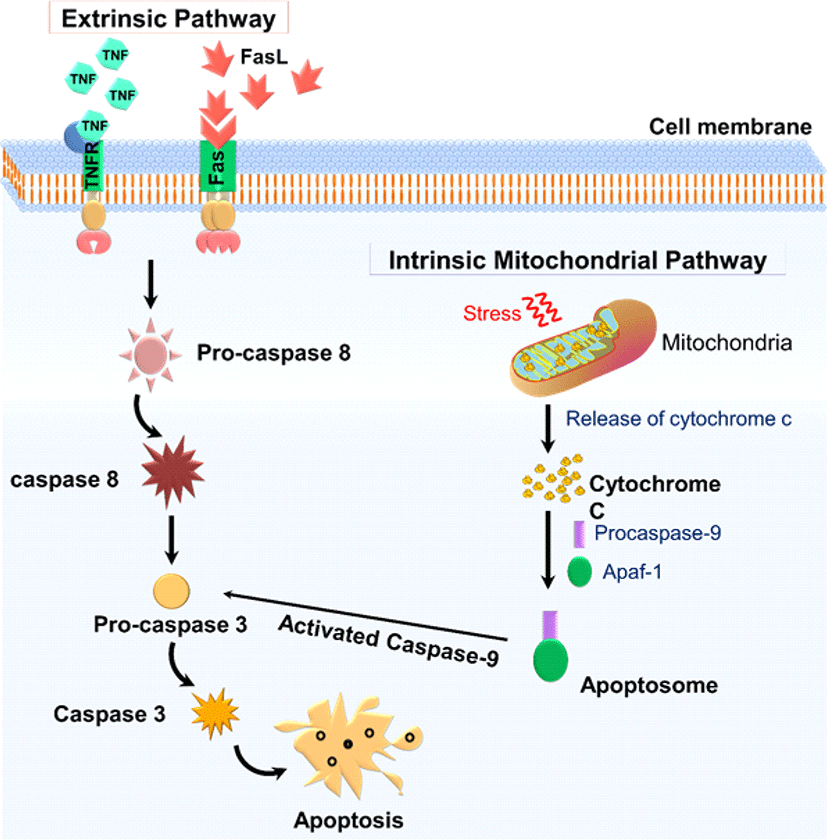
Apoptosis occurs primarily in two forms-intrinsic mitochondrial-mediated pathways and the pathway of extrinsic death receptors (Fig. 2). The extrinsic apoptotic pathway (death receptor-dependent) is initiated by the interaction of exposed death receptors of the cell surface belonging to the tumor necrosis factor receptor (TNFR) superfamily with their respective TNF family ligands. TNFR1-TNFα, FAS (CD95, APO1-FasL), TRAILR1 (death receptor 4; DR4)-TRAIL, TRAILR2 (death receptor 5; DR5)-TRAIL are the most widely characterized signaling systems of death receptor ligands [16]. Death ligand binding to the death receptor results in a binding site for an adapter protein, and the entire ligand-receptor-adapter protein complex is known as the death-inducing signaling complex (DISC), then begins assembling and activating pro-caspase 8. The activation of initiator caspases results in the processing of the downstream effector caspases-3, -6, and -7, the activation of which contributes to the cleavage of important cell viability substrates, contributing to cell death. The intrinsic pathway, also known as the mitochondrial pathway, requires several stimuli that act on multiple targets within the cell. Internal stimuli such as irreparable genetic damage, hypoxia, extremely high cytosolic Ca+ concentrations, and severe oxidative stress are some triggers for the initiation of the intrinsic mitochondrial pathway. Whatever the stimuli, this pathway is the result of increased mitochondrial permeability and the release into the cytoplasm of pro-apoptotic molecules such as cytochrome-c [17]. A group of proteins belonging to the family Bcl-2 tightly regulates this pathway. Bcl-2 family proteins are divided into two major classes, namely the pro-apoptotic proteins such as Bax, Bak, Bad, Bid, and Bik, and the anti-apoptotic proteins such as Bcl-2, Bcl-xL, Bfl-1, and Mcl-1. While the anti-apoptotic proteins control apoptosis by blocking the cytochrome-c release from mitochondrial, the pro-apoptotic proteins function by facilitating the release. Cytochrome-c binds to cytosolic Apaf-1 (activating apoptosis protease factor-1) and activates the development of a complex called apoptosome. That recruits the pro-caspase-9 initiator into its caspase recruitment domain. In turn, the process enables downstream caspases-3, -6, and -7 for the cleavage of cell substrates leading to apoptotic cell death [18].
With the essential goal in cancer therapy targeted towards apoptosis induction, diverse research has been focused and numerous reports have shown flavonoids are attractive agents capable of inducing apoptosis in cancer cells. Since flavonoids are generally non-toxic molecules, they could be useful in conjunction with toxic drugs commonly used in cancer therapy to reduce their toxic effects [19, 20]. In general, preclinical and epidemiological studies support the positive role of dietary flavonols, flavones, and isoflavones in ovarian cancer prevention. Phytochemicals have played a significant role in the prevention and treatment of cancer, but among all-natural polyphenol compounds, flavonoids, are a broad and diverse community [21, 22].
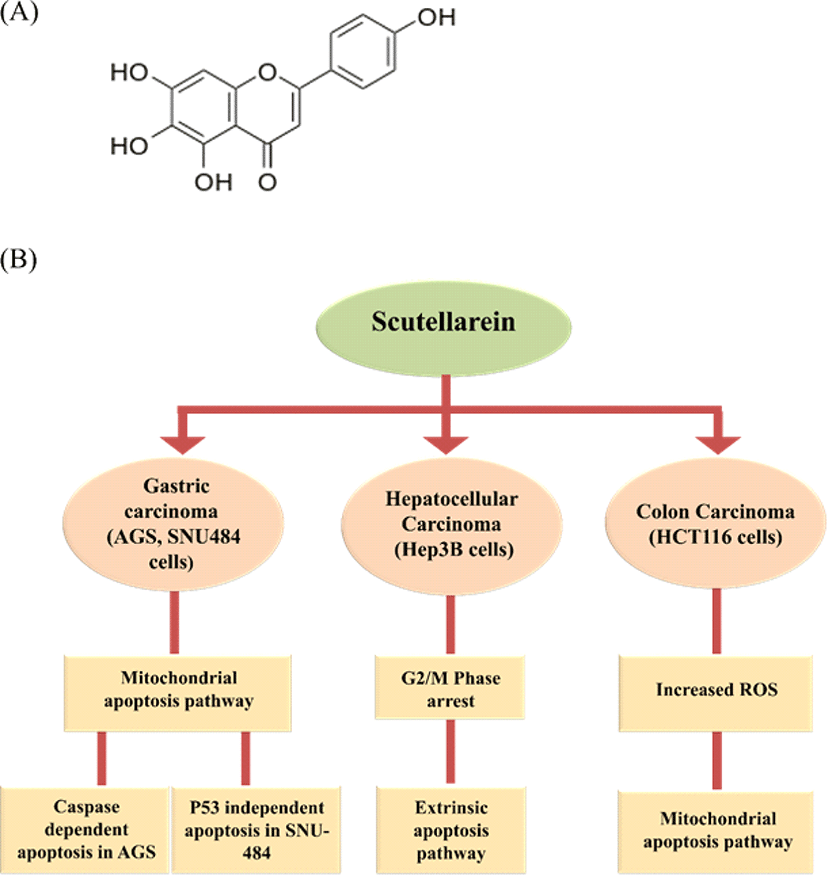
Scutellarein (5, 6, 7, 4’-tetrahydroxy flavone; Fig. 3A), a flavone, belongs to the flavonoids family. Scutellarein found in perennial herbs like Scutellaria baicalensis, Scutellaria lateriflora, Scutellaria barbata, Scutellaria baicalensis Georgi is a species of flowering plant in the Lamiaceae family. It is indigenous to several East Asian countries and the Russian Federation and has been cultivated in many European countries. The Chinese have used the dried root of this medicinal plant for over 2000 years as a traditional medicine known as Huang-Qin [23, 24]. Scutellarein found in Scutellaria baicalensis is considered a golden herb from Chinese medicinal plants has many pharmacological effects such as hepatoprotection, anti-bacterial, anti-viral, anti-tumor effects, antioxidant, ROS scavenger, anti-inflammation, anti-diarrheal and anti-convulsant [24]. Many studies have shown that Scutellaria baicalensis extract is cytotoxic to a broad range of cancer cells from humans, including brain tumor cells [25]. In one of our recent study, scutellarein-treated AGS and SNU484 (gastric cancer cell lines) showed inhibition in the cell proliferation. Further scutellarein induced G2/M phase arrest was observed and reported in SNU484 cells. Also, Scutellarein induced caspase-dependent apoptosis in AGS Cells and p53 independent apoptosis in SNU-484 Cells [26]. Scutellarein on Hep3B (hepatocellular carcinoma cell line) exhibited a concentration-dependent proliferation inhibitory effect. Scutellarein induces G2/M phase arrest by regulating CDC25C, Cdk1, and CyclinB1 protein expression. Further, it induced apoptosis through extrinsic apoptotic pathway with elevated levels of Fas and FasL in dose-dependent manner [27]. In addition, scutellarein extracted from Scutellaria barbata treated to HCT116 (human colon cancer cells), induced mitochondrial apoptotic pathway with ROS overproduction [28]. Overall, these studies confirm that scutellarein has a siginificant cytotoxic effect on cancer cells and can be used as a potent anti-cancer agent. We have illustrated the scutellarein induced apoptotic pathways in different cancer cell lines in Fig. 3.
Pectolinarigenin (5,7-Dihydroxy-6-methoxy-2-(4-methoxyphenyl) chromen-4-one; Fig. 4A) is a widely distributed flavonoid compound in a variety of medicinal plants, including Cirsium japonicum, Eupatorium odoratum, and Trollius chinensis [29]. Pectolinarigenin has demonstrated multifunctional bioactivities, including cytotoxic activity by inducing cell apoptosis, anti-inflammation, anti-allergy, and anti-tumor action. As a potential candidate for the treatment of human malignancies, it has drawn rising attention [30]. Cirsium japonicum belongs to the Compositae family and is commonly used in traditional Chinese medicine (TCM) for haemorrhage, hepatitis, hypertension, and blood circulation treatment [31].
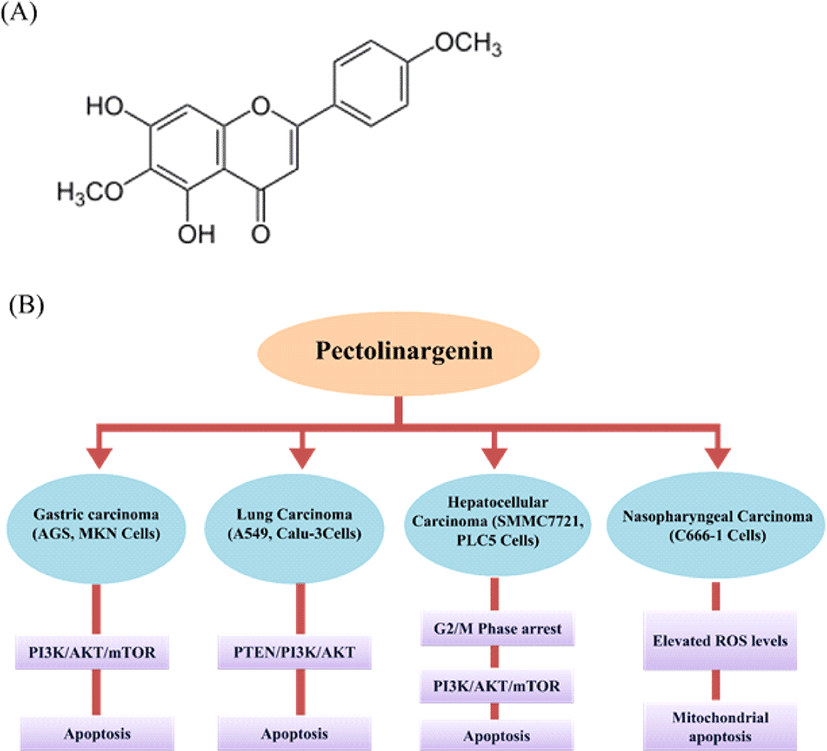
Pectolinarigenin’s cytotoxic activity was documented against MK-1 (human gastric adenocarcinoma), HeLa (human uterine carcinoma), B16F10 (human murine melanoma), GLC4 (human small lung carcinoma) and COLO 320 (human colorectal cancer) cell lines [32]. In one of our studies, pectolinarigenin exhibited AGS, MKN-28 (human gastric cancer cell line) growth inhibition and induced G2/M phase arrest with the expression of cell cycle regulators. Further, it also induced apoptosis and autophagy involving inhibition of PI3K/AKT/mTOR pathway [33, 34]. Pectolinarigenin treated with SMMC7721 and PLC5 (human hepatocellular carcinoma cell line) retarded the cell viability, proliferation, and cell invasion. It induced apoptosis, G2/M phase arrest, suppressed PI3K/AKT/mTOR pathway, and tumor growth in mouse xenograft models [30]. Pectolinarigenin inhibited the C666-1 (human nasopharyngeal carcinoma) cell proliferation and colony formation followed by induction of mitochondrial related apoptosis and elevated ROS levels [35]. Pectolinarigenin exhibited inhibitory effect on A549 and Calu-3 (human lung carcinoma cell lines) cell proliferation and induced apoptosis through PTEN/PI3K/AKT pathway [29]. Pectolinarigenin could be a new therapeutic anti-cancer agent to improve treatment responses for human gastric carcinoma with potential impact on the choice of therapy for PI3K/AKT/mTOR inhibitors, which are cancer specific. Overall, these studies confirm that pectolinarigenin can be used in cancer treatments. We have illustrated the pectolinarigenin induced apoptotic pathways in different cancer cell lines in Fig. 4.
Naringin is a disaccharide derivative that is (S)-naringenin substituted by a 2-O-(α-L-rhamnopyranosyl)-beta-D-glucopyranosyl moiety at position 7 via a glycosidic linkage (Fig. 5A). It is a dihydroxyflavanone, a member of 4′-hydroxyflavanones, a (2S)-flavan-4-one, and a neohesperidoside [36]. It is one of the main active components of Chinese herbal medicines, such as Drynaria fortune, Citrus aurantium L. and Citrus medica L. A comprehensive review of the literature showed that naringin has antioxidant, anti-inflammatory, anti-apoptotic, anti-ulcer, anti-osteoporotic, neuroprotective, anti-allergic, anti-dental caries, hepatoprotective, anti-diabetic and anti-carcinogenic properties [37–39].
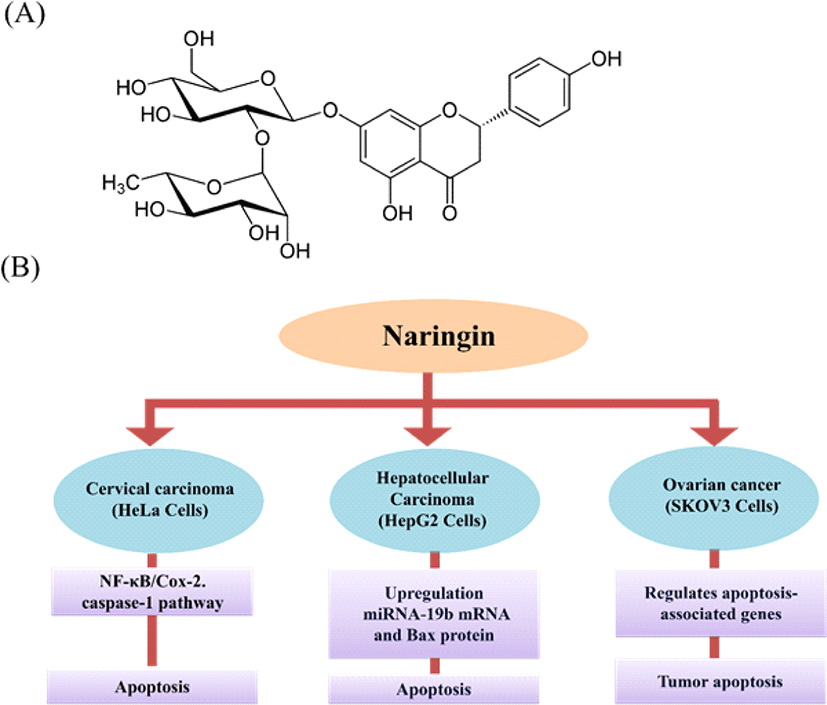
Naringin induces apoptosis in HeLa cells by the activation of the NF-κB-COX-2/caspase-1 pathway that contributes to cervical carcinogenesis, including cell apoptosis [40]. Naringin inhibits the SiHa (human cervical cancer cell) proliferation through cell cycle arrest at the G2/M phase of human cervical SiHa cells and apoptosis induction through the disruption of mitochondrial potential, and the activation of both intrinsic and extrinsic pathway [41].
Naringin inhibited cell growth in SKOV3 (human ovarian cancer cell line) and reduced tumor weight and size compared to control in vivo. In combinational therapy approach, naringin along with cisplatin promotes apoptosis in tumors dose-dependently and reduces cell growth-associated with gene expression [42]. Naringin treatment on HepG2 (human hepatocellular carcinoma) cells shows significant inhibition on cell growth and induction of apoptosis [43]. Investigation of the anti-cancer effect of naringin in thyroid cancer showed induction of apoptosis and suppression of PI3K/AKT pathway in thyroid cancer cell lines [44]. Overall, these studies confirm that naringin can be used as an anti-cancer drug. We have illustrated the naringin induced apoptotic pathways in different cancer cell lines in Fig. 5.
Conclusion and future perspectives
Cancer is a mind-boggling process that involves cell multiplication, and it remains a challenging condition that drives the cause of death. Conventional treatments are associated with many adverse drug reactions. So natural agents, including flavonoids, are generally considered safe in the treatment or prevention of diseases. While flavonoids have exhibited great potential in the battle against various cancers, this review provided an overview of some targeted flavonoids (scutellarein, peclinarigenin, naringin) and its apoptosis related proteins in the each cancer lines (Table 1). In future, more extensive studies about the potential use of flavonoids in targeting cancer cell apoptosis will be of great interest in the scientific community.
| Flavonoids | Cell lines | Intrinsic apoptosis pathway | Extrinsic apoptosis pathway | Apoptosis related protein | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bcl-xL | Cytochrome C | Bax | Fas | FasL | DR4 | Cl-PARP | Cl-caspase-3 | Cl-caspase-8 | Cl-caspase-9 | |||
| Scutellarein | HCT | - | ↑ | - | - | - | - | - | ↑ | - | - | [28] |
| AGS | ↓ | - | - | - | - | - | - | - | - | - | [26] | |
| SNU-484 | ↓ | - | - | - | - | - | ↑ | ↑ | - | ↑ | [26] | |
| Hep3B | - | - | - | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | - | [27] | |
| Pectolinarigenin | MCF-7 | - | - | - | - | - | - | ↑ | ↑ | - | - | [31] |
| A549 | ↓ | - | ↑ | - | - | - | - | - | - | - | [29] | |
| Calu-3 | ↓ | - | ↑ | - | - | - | - | - | - | - | [29] | |
| AGS | - | - | - | - | - | - | ↑ | ↑ | - | - | [33] | |
| MKN28 | - | - | - | - | - | - | ↑ | ↑ | - | - | [33] | |
| SMMC7721 | - | - | ↑ | - | - | - | - | ↑ | - | - | [30] | |
| PLC5 | - | - | ↑ | - | - | - | - | ↑ | - | - | [30] | |
| Naringin | Hela | - | - | - | - | - | - | - | ↑ | - | - | [40] |
| HepG2 | - | - | ↑ | - | - | - | - | - | - | - | [43] | |
| TPC | - | - | ↑ | - | - | - | - | ↑ | - | - | [44] | |
| SW1736 | - | - | ↑ | - | - | - | - | ↑ | - | - | [44] | |
