Introduction
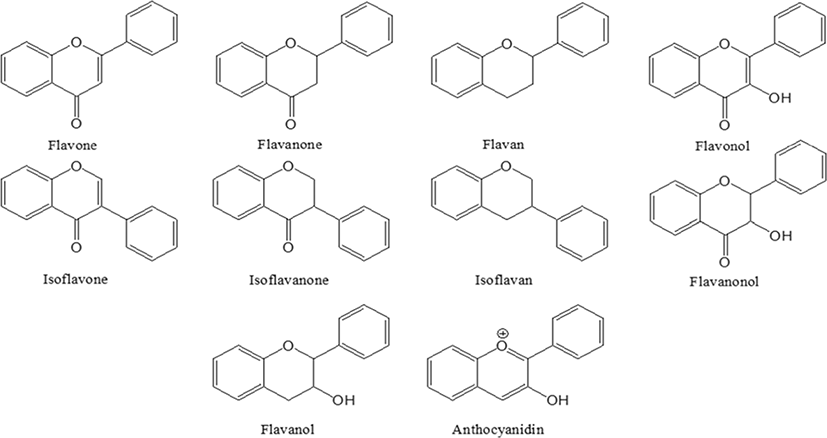
Researchers have shown an interesting trend in pharmaceutical development since the 21st century, to develop drugs from natural plant materials [1, 2]. The reason for the need of natural drugs is due to the limitations of expensive chemical drugs and its long-term side effects. Among the different secondary metabolites present natural materials, flavonoids are of importance due to their high biological benefits [3]. Flavonoids were named in the Latin word flavus, which means yellow, and is present in most plants [4, 5]. Flavonoids are known to be synthesized in certain parts of plants for a long time, providing color, fragrance and moisture to fruits and flowers, consequently helping to grow and develop seeds, spores and seedlings, and to protect against plaque [6, 7]. Flavonoids are natural compounds linked by three carbon chains, usually C6-C3-C6, and consist of an oxygenated heterocyclic ring (Fig. 1) [3]. The main classes of flavonoids are flavone, flavanone, flavan, flavonol, isoflavone, isoflavanone, isoflavan, flavanonol and anthocyanidin [8]. Natural flavonoids, especially their glucosides, are the most abundant polyphenols and more than 15,000 species are present in plants and are usually present in stems, flowers, leaves and fruits in the form of glycosides such as glucosides, galactoside, rhamnosides, arabinoside and rutinoside [9–11]. According to various reported studies, flavonoids have shown to exhibit anticancer [12–14], anti-diabetic [15–17], antioxidant [18–20], anti-inflammatory [21–23], and it also has been shown to play an important role in treating influenza [24], and managing obesity [25].
Flavonoids in plants play a preliminary role in protecting themselves from UV [26], frost and provide drought resistance, which has a beneficial effect on the plant on seasonal protection factors [27]. Flavonoids have a positive effect on human life as well in the treatment and prevention of various diseases. Flavonoids exhibit various roles in executing the antioxidant activity, metabolic regulation of carcinogens, and anti-inflammatory properties. In particular, the potential for preventing tumor cell proliferation is noteworthy [14]. Flavonoids prevent DNA damage from a variety of carcinogens, resulting in chemical prevention and protection [12], affecting chromatin structure contributing to both gene transcription and translation modifications [13]. Flavonoids show effective regulation of lipid metabolism by reducing the serum triglycerides, cholesterol, Low-density lipoprotein (LDL) and esterified fatty acids suggestive of anti-diabetic and anti-obesity activity [16]. Further, flavonoids are used as an agonist in various therapeutic strategies as anti-oxidants, cytoprotective and reported to treat inflammation and metabolism [19–21].
Among the different plant sources containing flavonoids, the genus Artemisia is a very diverse and widely distributed plant with more than 500 species belongs to the family of Asteraceae (Compositae), mainly distributed in temperate regions of Asia, Europe and North America. It is an herb with extensively distributed medicinal properties and also used for purposes like preparation of tea, and used in edible purposes like spices and ingredients in cooking [28]. The present review paper examines the three varieties of Artemisia: Artemisiaannua L., Artemisiaiwayomogi and Artemisiaargyi H and its functions in different therapeutic applications. Therefore, this review provides wide knowledge to the scientific community about the different flavonoids contained in the three species of Artemesia and their functions.
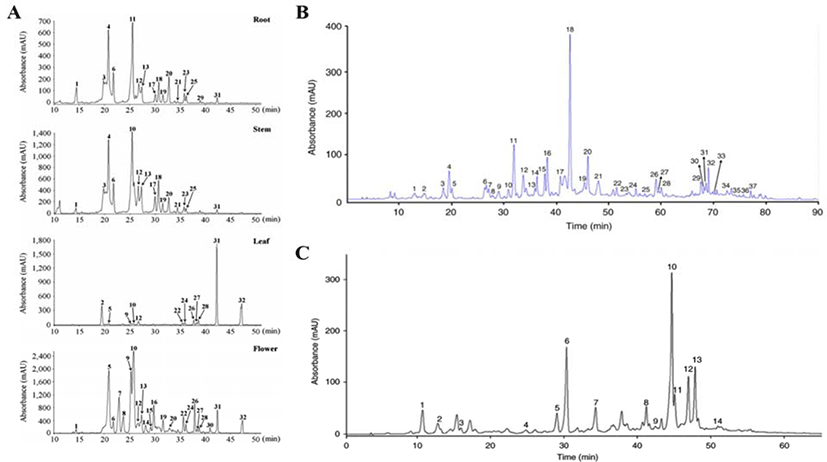
The total number of phenols and flavonoids contained in three species of Artemisia family were identified as 32 in Artemisia annua L., 37 in Artemisia iwayomogi and 14 in Artemisia argyi H., out of which flavonoids were 16 (Artemisia annua L.), 27 (Artemisia iwayomogi), 7 (Artemisia argyi H.) respectively (Fig. 2A–C). Flavonoids contained in three kinds of Artemisia were classified into 10 types according to the main structure, and functional type shown in Table 1. In addition, Fig. 2 represents the chromatogram obtained through HPLC analysis of the three species of Artemisia that has been completely studied [29–31].
| Name | Structure | Artemisia annua L. | Artemisia iwayomogi | Artemisia argyi H. | Function of classification | Remake |
|---|---|---|---|---|---|---|
| Quercetin |
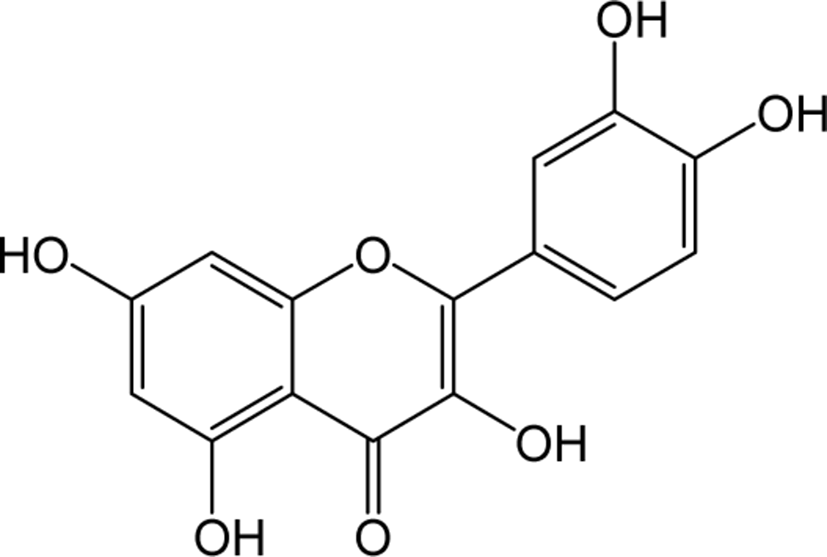
|
O | O | O | Flavonol | [41–47] |
| Kaempferol |
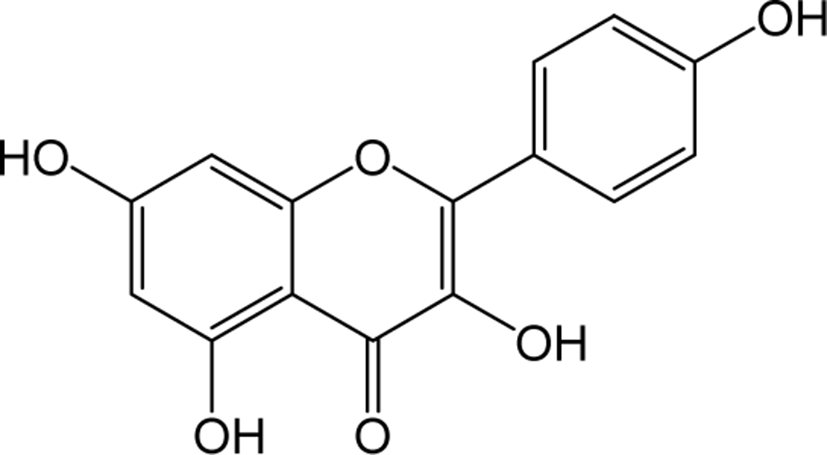
|
O | O | O | Flavonol | [48–51] |
| Rhamnetin |
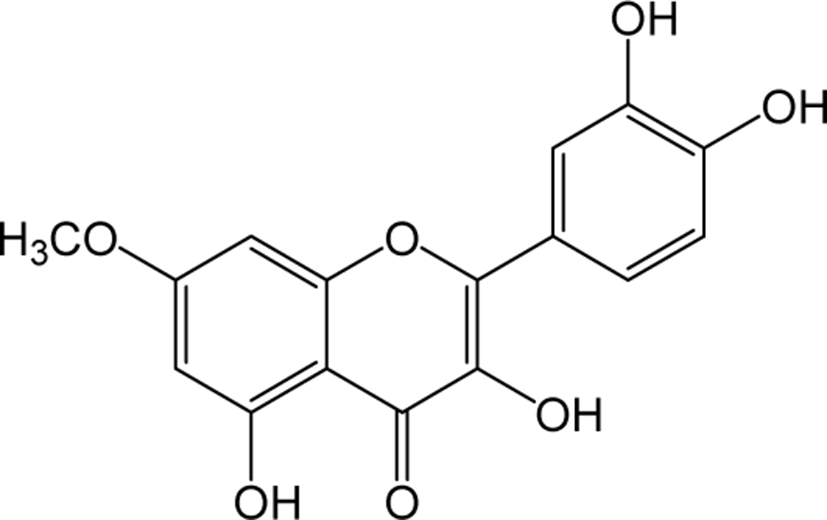
|
O | O | - | Flavonol | [52–55] |
| Diosmetin |
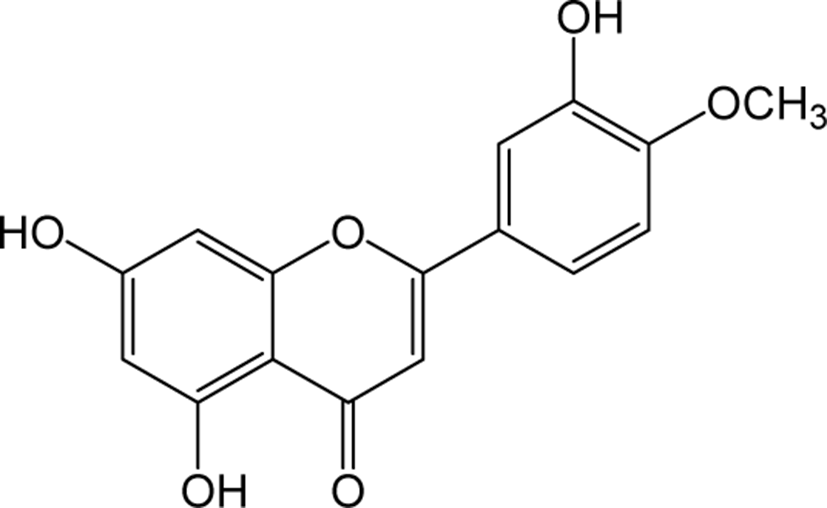
|
O | - | - | Flavone | [56–58] |
| Luteolin |
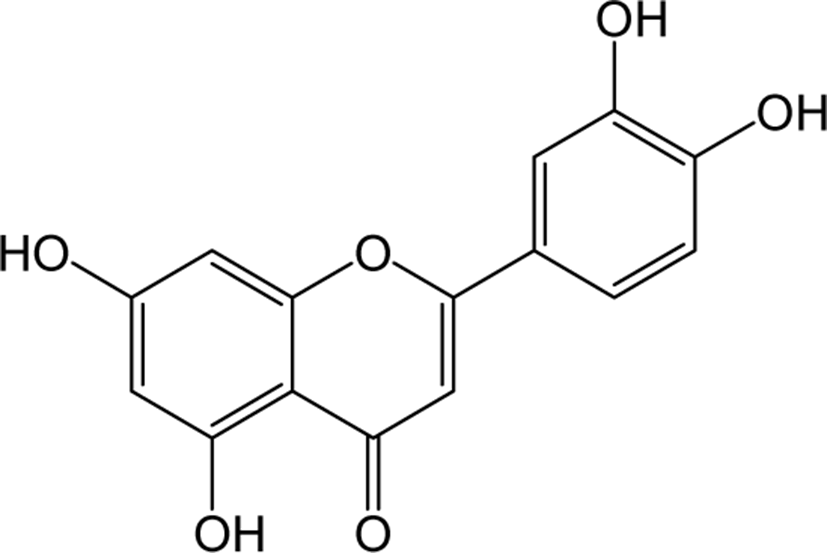
|
O | O | - | Flavone | [59–61] |
| Methoxy flavone |
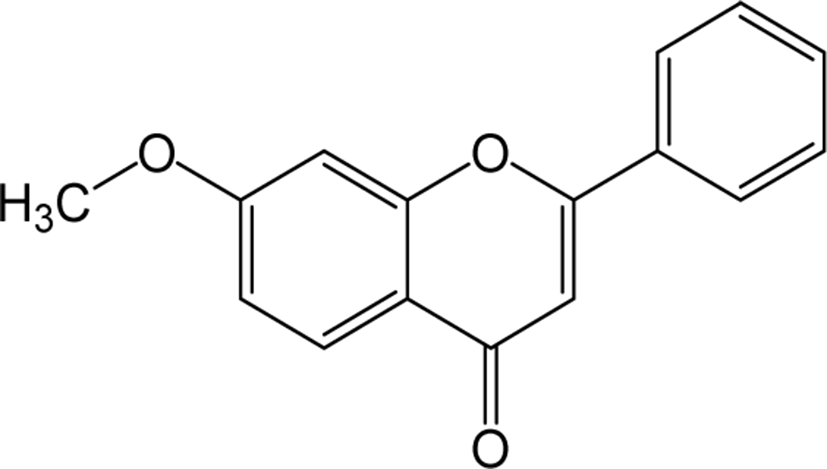
|
O | O | O | Flavone | [62–64] |
| Catechin |
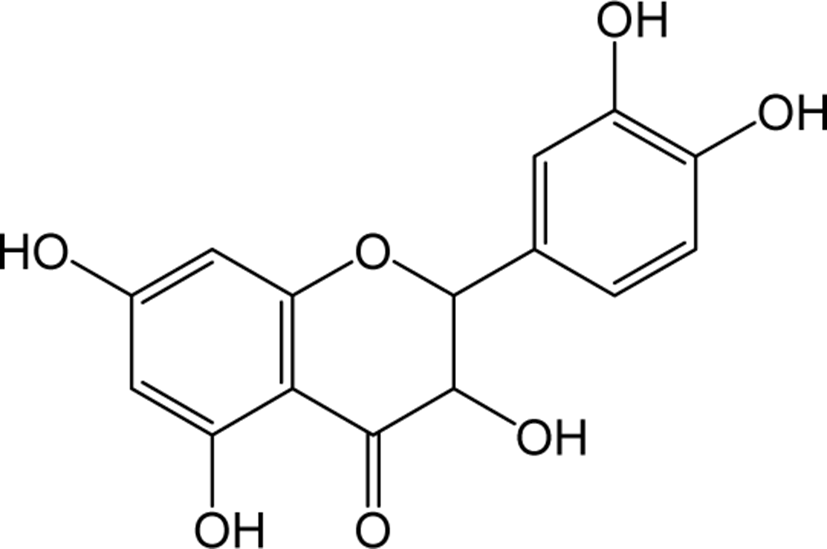
|
- | O | - | Flavanol | [65–75] |
| Apigenin |
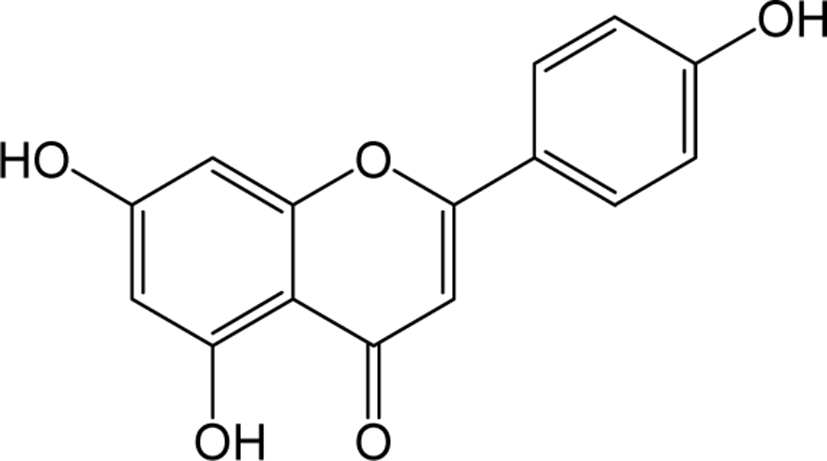
|
- | O | O | Flavone | [76–78] |
| Malvidin |
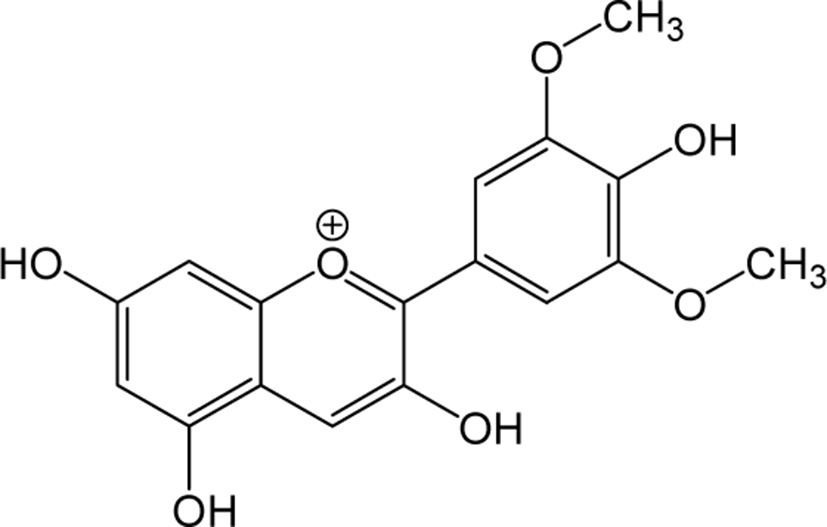
|
- | O | - | Anthocyanidin | [79–81] |
| Genkwanin |
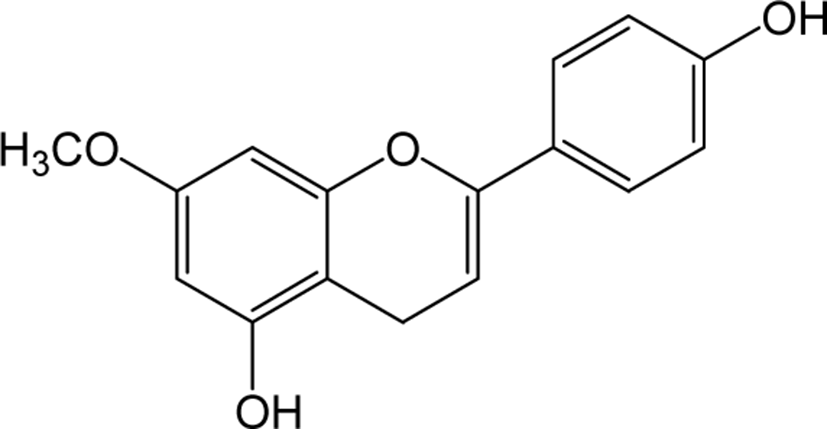
|
- | O | - | Flavone | [82–84] |
Artemisia annua L. grows into yellow flowers with a strong camphor scent in the month of September to an average height of 2 m [32]. Artemisia annua L. has been reported with a variety of activities including anti-inflammatory, antipyretic, anticancer, antifungal, antiparasitic, antiulcer, and cytotoxic effects [33]. Also, the essential oils and polyphenols contained in this plant were analyzed and proved to have excellent effects as antibacterial, antifungal and antioxidant activity [29, 30]. The AAP (A. annua polysaccharide), a water-soluble polysaccharide contained in Artemisia annua L. has been shown to inhibit cancer cell proliferation through p65-dependent mitochondrial signaling pathways via the activation of caspase-3 and -9, downregulation of Bcl-2 protein, upregulation of Bax protein, and release of cytochrome C from mitochondria [34]. Interestingly, in natural combinational therapy development using Artemesia, the interaction between three important components say arteannuin B, arteannuic acid, and scopoletin of artemisinin and A. annua showed a 2.6-fold improvement in mice with Plasmodium yoeli infection. This demonstrated the potential of certain components of A. annua could develop artemisin-based natural combination therapies for the treatment of malaria [35]. Collectively, the studies performed previously on Artemisia annua L. component and functions, it shows various beneficial activities that can be applied in the pharmaceutical industry.
Artemisia iwayomogi grows as flowers with yellow pigments during the month of August, the fruits ripen from September to October, and it grows up to an average height of 1 m tall. Artemisia iwayomogi is reported to exhibit biological effects such as antioxidants and anti-inflammatory and shown to treat immune-related disorders such as hepatitis and saccharide [30]. An essential oil present in Artemisia iwayomogi known as AIEO (Artemisia iwayomogi essential oil) has been shown to induce apoptotic death of Keratin forming tumor cell (KB cell line) mediated by mitogen-activated protein kinase (MAPK). AIEO does not show an only imbalance in the mitochondrial Bcl-2 and Bax proteins but also induced the activation of caspases and cleavage of poly ADP ribose polymerase (PARP) [36]. Similarly, a carbohydrate fraction of Artemisia iwayomogi known as AIP significantly reduced the levels of IgE and eosinophils in serum eosinophils followed by downregulation of inflammatory cytokines Th2 and TNF-α in lung inflammatory disease. [37]. A recent study on the phenol and flavonoid components of Artemisia iwayomogi was analyzed from its flower extract and demonstrated for its anti-inflammatory effect in LPS-induced macrophages by inhibiting the NF-kB signaling pathway [30].
Artemisia argyi H. is a perennial herb belongs to the class of Asteraceae, is widely distributed in most of the parts in Eastern Asia. Artemisia argyi H. has a higher composition of polyunsaturated fatty acids, total phenolic compounds, vitamin C and essential amino acids compared to other Artemisia varieties and has been reported to have an excellent radical scavenging effect [38]. Artemisia argyi is reported to exhibit a variety of biological activities, including antidiabetic, antioxidant, anticancer, anti-inflammatory, and antiallergic has been reported to have an excellent radical scavenging effect [39]. The flavone jaceosidin isolated from Artemisia argyi H. is known to inhibit the upregulation of COX-2 and MMP proteins, which are frequently expressed in various types of cancerous and transformed cells. [40]. The extract of Artemisia argyi H. show a protective effect by inhibiting the contrast-induced inflammatory response through activation of PPAR-γ (proliferator-activated receptor gamma), inhibition of MAPK phosphorylation and activation of caspase [39]. Polyphenols and flavonoids present in Artemisia argyi H. inhibits through the inhibition of nitric oxide production, nuclear factor-κB activation, mRNA expression of inducible nitric oxide synthase, tumor necrosis factor α and interleukin-1β, and phosphorylation of MAPKs in macrophages related to inflammation [31].
Quercetin belongs to the class of flavonol in flavonoids, which is rich in fruits, vegetables, leaves, seeds and grains, with a naturally bitter taste. Quercetin inhibits the inflammatory enzymes cyclooxygenase and lipoxygenase to reduce inflammatory mediators such as prostaglandins and leukotrienes and levels of inflammatory mediators such as NO synthase, COX-2 and CRP in human hepatocyte derived cell lines significantly [41, 42]. Quercetin is known to inhibit LDL oxidation, lower blood pressure, and prevents the development of cardiac hypertrophy [43, 44]. Quercetin also helps in the protection of membranes of red blood cells from the exposure to free radicals like tar in cigarette and prevent respiratory malfunctions [45]. Quercetin is also known to induce apoptosis and inhibit the growth of various cancer cells, and has been reported to be particularly effective in prostate cancer both in vitro and in vivo [46, 47].
Kaempferol belongs to the class of flavonol in flavonoids widely distributed as yellow crystalline solids. Kaempferol shown reported functions in reducing the production of reactive oxygen species (ROS), Malondialdehyde (MDA) and superoxide dismutase (SOD) activity in virus-induced inflammation through inhibition of the TLR4 / Myd88-mediated NF-kb and MAPK pathways [48]. Kaempferol also showed to regulate the normal plasma glucose, insulin, lipid peroxidation products, enzyme and non-enzyme antioxidants in diabetic rats [49]. The phosphorylation of mammalian targets of AKT (protein kinase B) and rapamycin (mTOR) signaling pathways has been shown to blocked by kaempferol treatment at the early stages of fast production and lowered early lipogenic factors such as C / EBPβ, KLFs in lipid accumulation conditions of adipocytes and in vivo zebrafish models [50]. Kaempferol also significantly inhibited the protein levels of DNA methyltransferase (DNMT3B) in ubiquitin-proteasome pathway to prevent premature degradation of protein synthesis [51].
Rhamnetin is a type of O-methylated flavonol that is found in a variety of plants and fruits. Rhamnetin has been reported to show anti-tumor activities in human breast cancer cells by the induction of apoptosis via the miR-34a / notch-1 signaling pathway [52]. Additionally, rhamnetin treatment on human prostate cancer cells LNCaP and PC-3 have been reported to result in increasing resistance to oxidative stress and inhibiting the cancer progression [53]. Rhamnetin also contributes to be effective compared to small molecular kinase inhibitors Sorafenib, etoposide and paclitaxel in treating hepatocellular carcinoma with susceptible to HepG2, ADR and MDR cells [54]. Rhamnetin has shown strong anti-tuberculosis activity and effectively inhibits lung inflammation by suppressing the mRNA levels of tumor necrosis factor-a, IL-6, IL-12 and matrix metalloproteinase-1 and terminate the IFN-y mediated stimulation of CRK1 and p38 mitogen activated protein kinase in MRC-5 lung cells [55].
Diosmetin is an O-methylated flavone and the aglycone part of the flavonoid glycosides diosmin occurs naturally in citrus fruits. Pharmacologically, diosmetin is reported to exhibit anticancer, antimicrobial, antioxidant, oestrogenic and anti-inflammatory activities [56]. Treatment with diosmetin on human prostate cancer cells significantly reduced the expression levels of cell cycle arrest markers cyclin D1, Cdk2, and Cdk4, accompanied by a decrease in apoptotic regulators c-Myc and Bcl-2 expression with an increase in Bax, p27Kip1 and FOXO3a protein expression. These results suggest the anti-cancer property of diosmetin in prostate cancer cells [57]. In addition, Diosmetin attenuates apoptosis in Sprague-Dawley rat retinal cells and in human retinal pigment epithelial (RPE) cells, and protects against diosmetin’s adverse effects on DNA damage and oxidative stress [58]. Apart from the anti-tumor effects, diosmetin has shown its therapeutic potential to improve renal damage by inhibiting the nuclear factor erythrocyte 2 related factor 2 / heme oxyenase-1 pathway [59].
Luteolin is a principal yellow dye compound that is obtained from the plant Reseda luteola, and has been used as a source of dye since the first millennium B.C. [60]. Aside of being a chromogenic agent, Luteolin also shows pharmacological action as anti-cancer agents in reported studies. Human liver cancer cell line SMMC-7721 treated with Luteolin shown to inhibit the cell viability, induced cell cycle arrest and induced apoptosis by increasing caspase 8 and decreasing bcl-2 expression.
In addition, luteolin also induced autophagic cell death by converting the protein LC3B-1 to LC3b-2 and increasing Beclin 1 expression in SMMC-7721 cells. Taken together, these results showed promising confirmations that luteolin can be used as an apoptotic inducer as well as an autophagic regulator in HCC treatment [61]. The anti-diabetic function of luteolin was identified in type 2 diabetes model KK-Ay mice which showed significant improvement in blood glucose, HbA1c, insulin and HOMR-IR levels respectively [62]. In vivo studies on memory impairment in streptozotocin (STZ) -induced Alzheimer’s rat models have been reported to significantly improve spatial learning and memory impairment by luteolin treatment [63].
Dimethoxyflavone is a group of flavone which is structurally composed of 14 hydrogen atoms, 17 carbon atoms and 4 oxygen atoms, and the name differs depending on their binding position. Inflammatory studies in LPS-induced peritoneal inflammatory mice supplemented with 6,7-trihydroxy-5-methoxyflavone reported being effective as an anti-inflammatory agent by reducing the concentration of inflammatory cytokines (TNF-α and IL-1β) [64]. A study on oral administration of MKE (K. parviflora rhizome) containing three methoxyflavones to dyslipidemic rats has been reported that the levels of cholesterol, triglycerides and LDL cholesterol were significantly decreased and HDL levels were significantly increased showing the hypolipidemic potential of methoxyflavones [65]. The antioxidant activity of DHMF (5,7-dihydroxy-8-methoxyflavone) has been studied in vivo upon oral administration in rats which showed the reversal of decreased activity of enzymes such as SOD, catalase and glutathione peroxidase. In addition, inflammatory mediators such as TNF-α and IL-1b induced by LPS have also been reported to be inhibited by DHMF [66].
Catechin belongs to the flavan-3-ol or simply the flavanol group, and is well-identified in various plants with the execution of a lot of significant activities as a secondary metabolite compound [67]. Catechin has shown to inhibit oxidative damage and reduces lipid peroxidation in vascular smooth muscle cells [68]. The anti-oxidant effects of catechin in mouse models has shown adverse effects by increasing the levels of enzymes SOD, catalase and glutathione peroxidase which plays an important role in ROS elimination [69]. Green tea containing high amounts of catechin has been reported to increase glutathione levels in plasma and tissues [70] and to participate in vitamin E, which supplements glutathione function [71]. In addition, catalase expression has been reported to increase in the aorta of hypertensive rats fed with green tea containing catechin for a period of two weeks [72]. Green tea catechin also showed to inhibit tumor growth and metastatic breast cancer mouse cells [73]. It also has an effect on reducing the formation of tumor blood vessels in breast cancer [74]. Notably, Epigallocatechin gallate increases the expression of p53, p21, and NF-kB, inducing apoptosis in vascular smooth muscle cells, preventing the development of atherosclerosis [75, 76]. Studies also suggest catechin inhibits the morphological and functional degeneration in the brain [77] and protects against accelerated memory degeneration in aged mouse models [78].
Apigenin belongs to the flavone group, which is an aglycone of glycosides found in many plants, and it appears as a yellowish solid crystal [79]. Apigenin inhibits the expression of TNF-α induced NK-kB activation which is responsible for the activation of transcription factors associated with COX2 and iNOS synthesis in inflammation of mouse macrophages [80]. Anti-tumor studies with apigenin in vivo reduced tumor growth and size in vivo with a lower Ki-67 index in tumor-induced mice. Results showed apigenin inhibits proliferation by inducing DNA damage, cell cycle arrest on G2 / M, p53 accumulation and apoptosis [81]. Apigenin also reduced endometriosis cell proliferation by inducing cell cycle arrest and apoptosis by regulating the ERK1 / 2, JNK and AKT cell signaling pathways [82].
Malvidin is a flavone glycoside primarily present in the pigment of plants, and it has potential functionality in protecting cells from oxidative stress and its related diseases [83]. Treatment with Malvidin inhibits H2O2-induced oxidative stress by blocking the lipid peroxidation and increased the cell viability in human fibroblast cells. In addition, the oxidative stress-related proteins COX-2 (Cyclooxygenase-2) and iNOS (inducible nitric oxide synthase) were downregulated [84]. Malvidin also significantly inhibited colony formation and induced apoptosis in HTC-116, colon cancer cells with the downregulation of cell cycle-related proteins in a concentration-dependent manner, leading to arrest of the G2 / M phase [85].
Genkwanin is a flavonoid belongs to the flavone group, which is a derivative of apigenin [86]. Genkwanin exerts an excellent anti-inflammatory effect by reducing the pro-inflammatory mediators such as iNOS, TNF-a, IL-1b, and IL6 without affecting the cytotoxicity of macrophages [87]. Genkwanin also showed downregulation of serum TNF-a, IL-6 and NO levels with reduction of foot swelling, arthritis index, inflammation of joint tissues and bone destruction in arthritis model rats. In addition, it has been reported to inhibit the activation of JAK / STAT and NF-kB signaling pathways in the synovial tissue of arthritis rats [88]. Genkwanin significantly inhibited cell proliferation in human colon colorectal cancer cells HT-29 and SW-480 and reduced inflammatory cytokine IL-8 secretion, and apparently improved visible gut histopathology was observed microscopically. These reports suggest the potential antitumor activity through enhancing the immune function by the reduction of inflammatory cytokines [89].
Conclusion
In summary, this review paper examines three species of Artemisia native to Korea (Artemisia annua L., Artemisia iwayomogi, Artemisia argyi H.). In brief, the flavonoids contained in three Artemisia varieties examined by chromatography and classified into 12 types according to the main structure are reported based on studies. Subsequently, the physiological activities of the classified flavonoids were quoted on their various activities such as antioxidant, anti-inflammatory, antiviral, anti-cancer, metastasis suppression and anti-obesity.
Thus, the review gives a extensive knowledge on the three native varieties of Artemisia in Korea and their therapeutic values in the prevention and treatment of various diseases which aids in research for the development of naturopathies using Artemisia.