Introduction
According to Ronco and colleagues, cardiorenal syndrome (CRS) is associated with cardiac and renal disease and is defined by “acute or chronic dysfunction in one organ that may induce acute or chronic dysfunction of the other” [1]. The interaction between the cardiac and renal systems is important in determining blood pressure and blood volume, both of which play a role in the vasomotor system and fluid balance. CRS occurs as a result of disparities in correlations between these.
In human medicine, CRS is classified by five categories: 1) acute cardiorenal, 2) chronic cardiorenal, 3) acute renocardiac, 4) chronic renocardiac, and 5) secondary CRS; this distinction was adopted by veterinary medicine, being termed cardiovascular-renal axis disorder (CvRD) [2]. The correlation of CRS is caused by the renin angiotensin aldosterone system (RAAS), cardiac output, glomerular filtration rate (GFR), and systemic hypertension [3]. In veterinary medicine, CvRD lacks pathologically and etiologically specific data, but shares the common pathophysiological patterns of CRS and CvRD in humans [3]. CvRD has been demonstrated in a number of studies in dogs [4, 5], and similarly been seen in cats [6]. As a result, CvRD is termed as structural or functional damage caused by diseases of the heart or kidneys, or toxins or drugs, resulting in the disruption of normal interaction between these organs and the destruction of one or both organs [3].
Telmisartan is a drug belonging to the angiotensin receptor blocker (ARB) family, which can respond to the activation of the RAAS by angiotensin escape that occurs with the use of angiotensin converting enzyme inhibitor (ACEi), and has proven to be useful for suppressing proteinuria in cats [7]. It is also recommended for systemic hypertension and proteinuria in cats with chronic renal failure [8, 9].
The aim of this study is to compare long term changes of various indicators, including hypertension and proteinuria, before and after administration of telmisartan in cats with CvRD.
Materials and Methods
The study was conducted in 20 cats (5 spayed females and 15 castrated males, weighing 4.0–7.6 kg) with CvRD with no other disease, aged between 4 and 18 years (mean 10.4 ± 4.6).
Cats participating in this study were selected according to several criteria. For study probabilities, cats with diseases other than CvRD, such as hyperthyroidism or pancreatitis, were excluded. The selection criteria were those who were clinically diagnosed with CvRD, with one of the two following criteria: heart disease with hypertrophic cardiomyopathy (HCM) or chronic kidney disease (CKD). For heart disease with HCM, echocardiograms were selected based on the criteria that the size of the left atrium (LA) was more than 15 mm in the right parasternal long axis 4 chamber view [10] and that the thickness of the myocardial muscle was 6 mm or more in the short axis papillary muscle view [11, 12]. Criteria for heart disease with CKD was in accordance with the International Renal Interest Society (IRIS) stages [13], while meeting one or more of the following criteria: cats with CKD at 2–4/4 grades with serum creatinine concentrations above 1.6 mg/dL, Urine protein to creatinine (UP/C) ratio readings>0.2 [14], and hypertensive systolic BP>150 mmHg on Doppler [9, 15]. In addition, the owner had to be present, and individuals weighing less than 2 kg were excluded.
Before and after telmisartan (Semintra® oral solution, Boehringer Ingelheim, Mexico) administration (1 mg/kg, PO, SID), patients were followed 4 times at 30-day intervals for a total of 90 days. Body weight, serum chemistry (IDEXX Catalyst DxTM Analyzer, IDEXX Laboratories, USA), total cell blood count (CBC; IDEXX Procyte DxTM Hematology Analyzer, IDEXX Laboratories, USA), urine specific gravity (USG), urine pH, and UP/C ratio (IDEXX Catalyst DxTM Analyzer, IDEXX Laboratories, USA) tests were performed at each visit over the course of the follow up period.
Blood pressure was measured using a Doppler device (Parks Model 811-B, Parks Medical Electronics, USA) according to the consensus guidelines of the International Society of Feline Medicine [9]. Before being examined, cats rested calmly for 5–10 minutes in the examination room. The placement and size of the inflatable cuffs were determined by measuring the forelimbs with the short length of the cuff to be 30%–40% of the forelimb length. The characteristic ultrasonic sound of Doppler devices can cause tension in cats, so headphones were used. After ultrasonic gel was applied to the leg region to be measured and the transducer closely attached, a sphygmomanometer was used to inflate the cuff to a pressure of 20–40 mmHg until no Doppler ultrasound signals generated by red blood cells (RBCs) could be heard. When no sound was heard, the sphygmomanometer was deflated until the sound could be heard again, and at the first moment of Doppler ultrasound detection systolic blood pressure (SBP) was measured.
Statistical analyses were performed using commercially available statistical software (Jamovi ver 1.0.0 for Mac). Continuous variables are presented as mean ± SD. Differences in the various indices that change with time before and after administration of telmisartan were assessed using Repeated Measures ANOVA (non-parametric). In all comparisons, p<0.05 was considered statistically significant, unless otherwise stated.
Results
The breeds of cats included in this study were as follows: Domestic Short Hair (10/20, 50.0%), Himalayan (1/20, 5.0%), Persian (2/20, 10.0%), Russian Blue (3/20, 15.0%), Siamese (2/20, 10.0%), and Turkish Angora (2/20, 10.0%). The Domestic Short Hair was overrepresented in this study.
As measured by CBC analysis, no significant changes in RBCs or white blood cells (WBCs) were found. Serum chemistry analysis revealed that elevation of creatinine and blood urea nitrogen (BUN) (azotemia), was not statistically significant. In analysis of the liver, there were no significant changes in alanine aminotransferase (ALT), alkaline phosphatase (ALKP) and BUN. No significant changes in hematocrit (HCT) or total protein (TP) related to dehydration were found. As measured by urinalysis, changes in urine pH and USG were not significant. However, although not statistically significant, USG, as well as body weight, was slightly increased (p = 0.912 and p = 0.991, respectively).
UP/C ratio (p<0.001) decreased significantly over time after administration of telmisartan. Before telmisartan administration, the UP/C ratio was 0.39 ± 0.255 (Day 0), and 0.29 ± 0.056 on the 30th day (Day 30), 0.28 ± 0.040 on the 60th day (Day 60), and 0.20 ± 0.128 on the 90th day (Day 90) after administration, respectively. The UP/C ratio was significantly lower in cats with CrVD over time, at each time point from Day 0 to Day 90, at 30 day intervals (Fig. 1A).
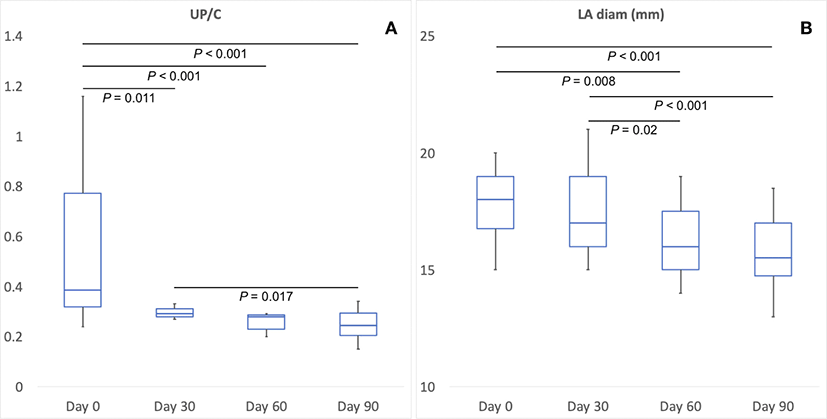
Echocardiographic parameters were decreased in the LA and Interventricular septum end-diastolic diameter (IVSDd), but the left atrial diameter was significantly different (p = 0.011) and the IVSDd (p = 0.381) was not. LA diameter was 17.9 mm ± 1.6 before telmisartan administration (Day 0), and 17.4 mm ± 1.8 on the 30th day (Day 30), 16.1mm ± 1.6 on the 60th day (Day 60), and 15.7 ± 1.7 on the 90th day (Day 90) after the administration, respectively. LA diameter was significantly lower in cats with CvRD over time, at each time point from Day 0 to Day 90, at 30 days intervals (Fig. 1B).
Differences in the various indices that changed over time, before and after drug administration in this study population, are summarized in Table 1.
SBP, systolic blood pressure; HCT, hematocrit; RBC, red blood cell; WBC, white blood cell ALKP, alkaline phosphatase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; Crea, creatinine; TP, total protein; UP/C, urine protein to creatinine ratio; SG, specific gravity; LA, left atrium; IVSDd, interventricular septum end-diastolic diameter.
Discussion
This study demonstrated a clear and gradual decrease in the UP/C ratio in cats with CvRD, after administration of telmisartan. Although blood pressure and UP/C ratio were decreased in our previous study of telmisartan administration for CKD in cats [16], this study showed a decrease in UP/C, but no significant change in systemic blood pressure. In addition, although telmisartan is known to have a relatively higher liver clearance than renal clearance [17], no significant changes in serum liver or kidney profiles (albumin, ALKP, ALT, BUN, creatinine) were observed in this study, similar to our previous study [16].
The evaluation of proteinuria for 24 hours and quantitative evaluation would have been inconvenient for the study participants and is also a troublesome procedure. Therefore, we performed the UP/C test, which is a gold standard method for evaluating proteinuria through one-time sampling [18], and results are very similar to the 24-hour quantitative test method [19, 20]. Studies on small animals, including dogs and cats, have shown that proteinuria is closely related to patient survival [14, 21–29]. In addition, since proteinuria is closely related to kidney disease [30–33], controlling proteinuria in CvRD patients could be a strategy for increasing survival rates [14, 29].
All cats participating in the study were subjected to cystocentesis through an ultrasound guide to collect urine. Therefore, macro hematuria did not occur, but micro hematuria due to blood collection occurred, which caused an occult blood reaction that did not affect the UP/C test [34]. There were no significant differences between urine pH and USG, suggesting that the effect of telmisartan in cats with CvRD was due to vasodilation, rather than chemical changes in the urine. This phenomenon is very similar to a previous study [16] that observed the effect of temisartan in CKD patients.
A decrease in blood pressure, which was significant in a previous study [16], was not found in this study. This suggests the phenomenon is caused by the compensatory mechanisms of cardiac output due to hemodynamic changes caused by HCM, not by the presence of CKD alone, or by the possibility of blood pressure measurement errors that frequently occur in cat patients.
Echocardiography showed that the size of the LA was reduced. Since HCM in cats is a disease characterized by atrial enlargement, these results are very interesting. The reduction in size of the LA due to the administration of ARBs, such as telmisartan, is a positive therapeutic outcome for cats with CvRD. However, IVSDd, which signifies the thickness of the ventricular septum at the time of diastole, decreased over time, but no significance difference was found. There are several limitations to this study. First, there is a low number of research population. A study with a larger research population will be necessary to derive more meaningful results. In addition, the lack of follow-up of other items, besides IVSDd, in echocardiography is the second limitation of our study.
In conclusion, oral administration of telmisartan to cats with CvRD is effective in improving proteinuria, which is a positive aspect of long-term survival in cats with CvRD.