Introduction
Worldwide, coronary artery disease (CAD) is the principal cause of death among patients [1]. Vascular smooth muscle cell (vSMC) proliferation, commonly known as neointima hyperplasia, is a major side effect after balloon angioplasty and/or coronary stent implantation for treating patients with obstructive CAD [2]. Dyslipidemia is a well-known risk factor for cardiovascular disease. vSMC proliferation in severe hypercholesterolemia has already been studied [3, 4]. However, there has been no study on the effects of mild elevation in serum cholesterol level on vSMC proliferation after vessel injury in a large animal model such as a porcine model.
Therefore, the present study aimed to evaluate the effect of mild elevation in serum cholesterol level on vSMC proliferation in a porcine coronary overstretch restenosis model using a balloon angioplasty catheter or drug-eluting coronary stent.
Materials and Methods
The animal study was approved by the Ethics Committee of Chonnam National University Medical School and Chonnam National University Hospital (CNU IACUC-H-2016-04) and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Pigs were divided into two groups according to the diet they were fed: commercial normal diet (CND; 3.6% fat content; n = 4) and a high-fat diet (HFD; 10.2% fat content; n = 4). The diets were fed at day 0 before coronary artery injury was induced using balloon angioplasty or stent implantation (day 7) and continued for the entire duration of this experiment for 35 days (Fig. 1).
Whole blood samples were collected from pigs at baseline (before diet administration, day 0), immediately after balloon angioplasty or stent implantation (day 7), and at follow-up and sacrifice (day 35). Serum cholesterol levels were measured on days samples were collected (Alere Cholestech LDX® System, Abbott, Santa Clara, CA, USA; Fig. 1).
Study animals were castrated male pigs weighing 20–25 kg. To prevent acute thrombosis after balloon angioplasty or stent implantation, premedication with aspirin 100 mg and clopidogrel 75 mg per day was administered for 7 days before the procedure. On the day of the procedure, pigs were anesthetized with zolazepam and tiletamine (2.5 mg/kg, Zoletil50®, Virbac, Caros, France), xylazine (3 mg/kg, Rompun®, Bayer AG, Leverkusen, Germany), and azaperone (6 mg/kg, Stresnil®, Janssen-Cilag, Neuss, Germany). Supplemental oxygen was continuously provided through an oxygen mask. Subcutaneous 2% lidocaine at the cut-down site was administered; the left carotid artery was surgically exposed, and a 7-Fr sheath was inserted.
Continuous hemodynamic and surface electrocardiographic monitoring was performed throughout the procedure. Then 5,000 U of heparin was intravenously administered as a bolus prior to the procedure; the target coronary artery was engaged using standard 7-F guide catheters, and control angiograms of both coronary arteries were performed using a nonionic contrast agent in two orthogonal views.
The balloon catheter or coronary stent was deployed by inflator, and the resulting balloon or stent-to-artery ratio was 1.3:1. Coronary angiograms were obtained immediately after balloon angioplasty or stent implantation. All equipment was then removed, and the carotid artery was ligated.
Four weeks after balloon angioplasty or stent implantation, follow-up angiography was performed in the same orthogonal views previously used; the animals were then sacrificed by an intracoronary injection of 20 mL of potassium chloride.
The hearts were removed, and the coronary arteries were pressure-perfusion fixed at 110 mmHg in 10% neutral buffered formalin overnight. Step sectioning of each artery was performed, followed by routine processing under light microscopy and staining for histological analysis.
Histopathological evaluation of each artery was performed by an experienced cardiovascular pathologist. The specimens were embedded; sections of 3–4 μm thickness at approximately 1 mm apart were obtained and stained with hematoxylin-eosin for histological analysis. Measurements of the histopathological sections were performed using a calibrated microscope, digital video imaging system, and microcomputer program (Visus 2000 Visual Image Analysis System, IMT Tech, CA, USA). Borders were manually traced for lumen area, area circumscribed by the internal elastic lamina, and innermost border of the external elastic lamina (external elastic lamina area). Morphometric analysis of the neointimal area for a given vessel was calculated as the measured internal elastic lamina area subtracted by the lumen area. The measurements were performed for five cross-sections from the proximal and distal ends and the three midpoints of each stented segment. Percent area stenosis was calculated as follows: 100 × [1 – (lesion lumen area/lesion internal elastic lamina area)] [5]. All results were interpreted by two independent pathologists in a blinded manner.
Arterial injury at each strut site was determined by anatomical structures penetrated by each strut. A numerical value was assigned as follows [5]: 0 = no injury; 1 = break in the internal elastic membrane; 2 = perforation of the media; and 3 = perforation of the external elastic membrane to the adventitia. The average injury score of each segment was calculated by dividing the sum of the injury scores by the total number of struts at the examined section.
Regarding the inflammation score for each individual strut, the grading was as follows: 0 = no inflammatory cells surrounding the strut; 1 = light, noncircumferential lymphohistiocytic infiltrate surrounding the strut; 2 = localized, moderate-to-dense cellular aggregate surrounding the strut noncircumferentially; and 3 = circumferential dense lymphohistiocytic cell infiltration of the strut. The inflammation score of each cross-section was calculated by dividing the sum of the individual inflammation scores by the total number of struts at the examined section [6]. Ordinal data for fibrin were collected for each stented section using a scale of 0 to 3 [7].
Results
Coronary artery injury using balloon catheter inflation was induced in the left anterior descending and left circumflex artery in 2 pigs (1 from each group). Two coronary stents were implanted in two coronary arteries in each pig. A total 12 stents, including 6 in the HFD group and 6 in the CND group, were placed in the middle to proximal left anterior descending and circumflex artery in 6 pigs (3 in each group). Mortality rate was zero until sacrificed in this study.
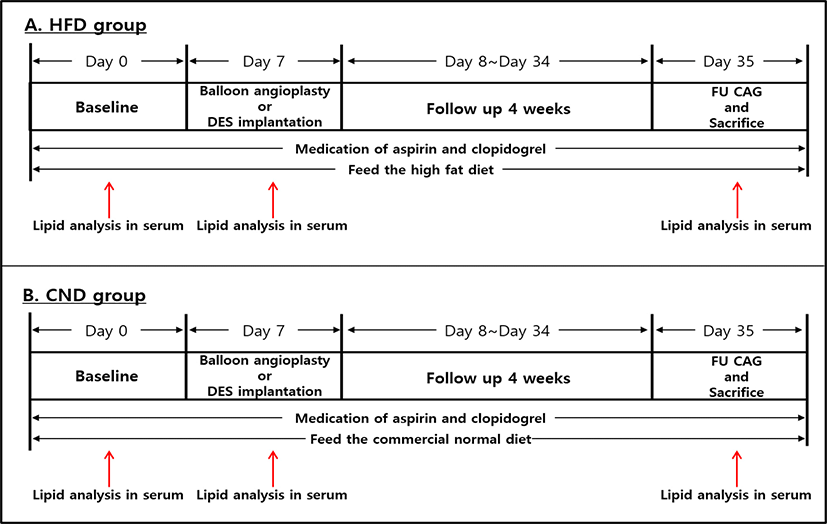
At 1 and 5 weeks after feeding, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride levels were significantly higher in the HFD group than in CND group (Fig. 2).
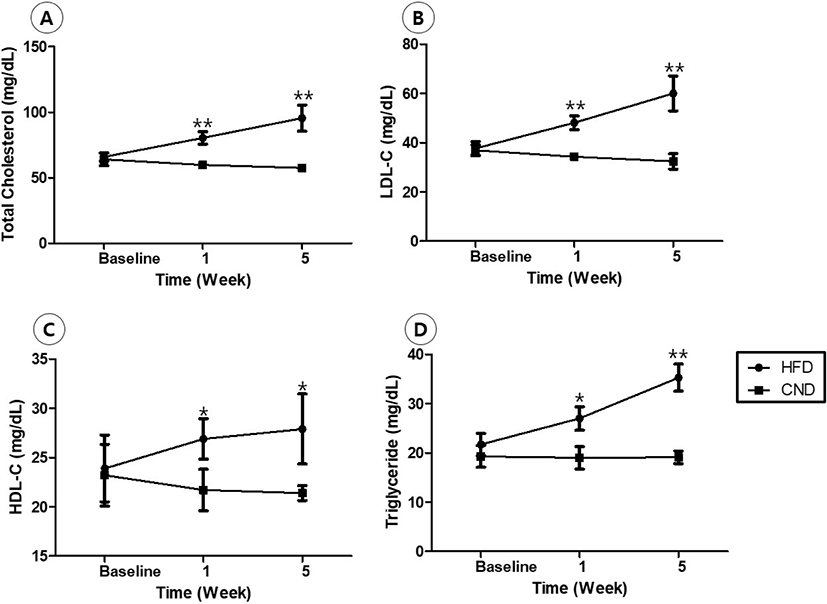
The lumen area was significantly lesser in the HFD group (1.8 ± 0.51 mm2 in HFD group vs. 2.8 ± 0.32 mm2 in CND group, p<0.01; Fig. 3). Internal elastic lamina area, neointimal area, and percent area stenosis could not be measured because the intima line was not clear.
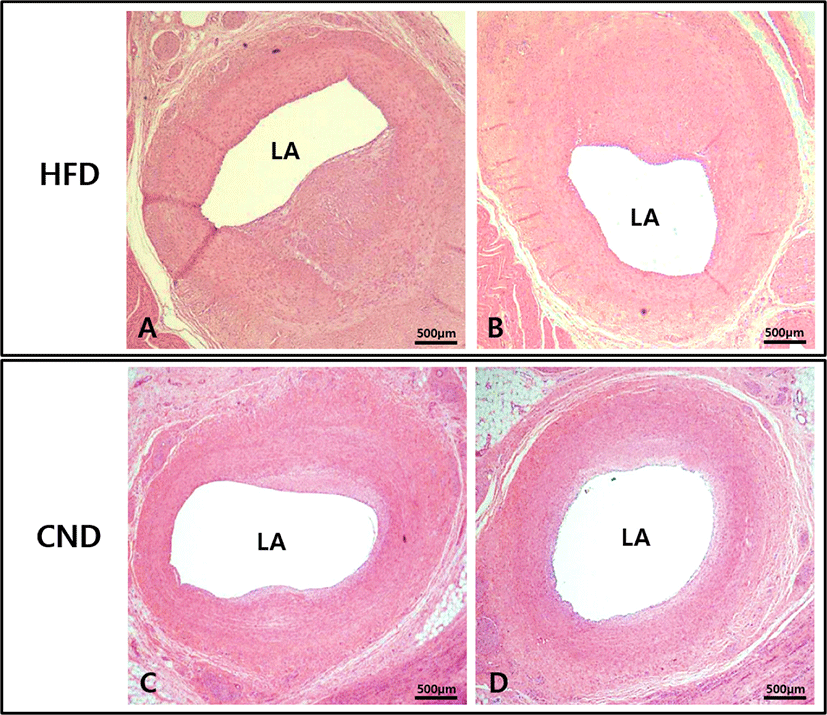
There were no significant differences in the injury score (1.3 ± 0.49 in the HFD group vs. 1.3 ± 0.52 in the CND group, p = NS) and internal elastic lamina area (5.3 ± 0.41 mm2 in the HFD group vs. 5.3 ± 0.42 mm2 in the CND group, p = NS) between the two groups. There were significant differences in the neointimal area (3.3 ± 0.34 mm2 in the HFD group vs. 2.7 ± 0.33 mm2 in the CND group, p<0.05), lumen area (2.0 ± 0.33 mm2 in the HFD group vs. 2.6 ± 0.54 mm2 in the CND group, p<0.05), fibrin score (1.1 ± 0.38 in the HFD group vs. 1.2 ± 0.41 in the CND group, p = NS), inflammation score (1.6 ± 0.53 in the HFD group vs. 1.5 ± 0.55 in the CND group, p = NS), and percent area stenosis (62.4 ± 5.15% in the HFD group vs. 52.0 ± 7.96% in the CND group, p<0.05) between the two groups (Fig. 4, Fig. 5A, B, C).
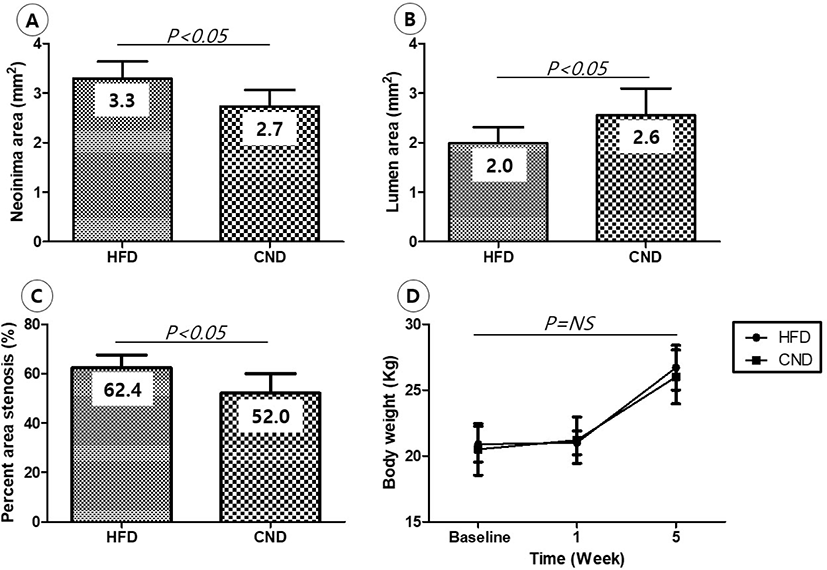
There was no difference in body weight between the two groups until the end of the experiment (Fig. 5D).
Discussion
Our study was conducted to investigate the effect of a mild elevation in serum cholesterol level on vSMC proliferation in a porcine coronary overstretch restenosis model. Our study demonstrated that the mild elevation in serum cholesterol level promoted neointimal hyperplasia after vascular injury in the HFD group.
Previous studies have shown that the consumption of a high-cholesterol and/or HFD is associated with an increase in the prevalence of vascular diseases [8–10]. Hypercholesterolemia is one of the most important risk factors for cardiovascular diseases.
A large animal model such as a porcine model provides enables researchers to physiologically, pathologically, and preclinically understand diseases. Based on this, knowledge of the prevention or inhibition of the progression of diseases is gained. These models are also important tools for evaluating novel therapeutic drugs and medical devices. In previous studies, the degree of vSMC proliferation after vessel injury in pigs was consistent with the degree in human trials [11, 12]. In addition, pigs have similarities to humans in terms of coronary artery anatomy, vessel physiology, disease progression, lipoprotein profile, and lipid metabolism [13–16]. Therefore, our findings can be considered to be applicable in humans.
The main difference of our study from previous studies is the timing of vessel injury. Previous studies using HFD-induced hypercholesterolemia models have been conducted where serum cholesterol level rise to their peak and then balloon angioplasty or stent implantation was performed to induce injury [17–20]. One more important difference is that cholesterol levels did not exceed the standard limit [21].
Coronary arterial injury induced by either balloon angioplasty or stent implantation leads to neointimal hyperplasia of intimal smooth muscle cells in a porcine model [22]. There was a significant correlation between arterial injury and vSMC proliferation [23]. It is necessary to confirm the intima layer of the injured arteries to determine the injury score. It was more difficult to accurately measure the injury score after balloon angioplasty than after stent implantation when injury was induced. After stent implantation, the exact injury score can be measured based on the position of the stent strut in the vessel wall. If there is no difference in the injury score between the two groups, vSMC proliferation may be a useful histopathological comparison criterion. Therefore, we compared the lumen area in each after balloon angioplasty.
This study has some limitations. First, hematoxylin and eosin staining was used for estimating vSMC proliferation after injury was induced. Immunohistochemistry techniques using a specific antibody such as α-smooth muscle actin is the gold standard for estimation [24]. Second, we did not determine the complete blood count during the systemic inflammatory reaction in hypercholesterolemia [25, 26]. Third, this was not a long-term study. Finally, the sample size was small.
However, our results showed that mild elevation in serum cholesterol level was related to a higher degree of vSMC proliferation.
This study demonstrated that patients with cardiovascular disease with a mild elevation in serum cholesterol level requires a more aggressive treatment for cholesterol reduction. In particular, the serum cholesterol levels of patients undergoing percutaneous transluminal coronary angioplasty using balloon angioplasty and coronary stenting should be carefully managed.