Introduction
Vascular malformations have been reported in about 20% of dogs and cats. Most congenital anomalies are not clinically significant. Patent ductus arteriosus (PDA) is one of most common congenital cardiac vessel malformations in dogs [1]. Persistent left cranial vena cava (PLCVC) accounts for 45% of the clinical cases of congenital anomalies of the great vessels in dogs. Blood vessels function in the fetus but deteriorate as the embryo matures. Rarely, the chest cavity vessels fail to deteriorate. PLCVC normally atrophy and the left caudal cardinal veins become the coronary sinus [2]. However, PLCVC can develop abnormally when the left caudal cardinal veins do not atrophy, leaving a connection between the left common cardinal vein and the coronary sinus [3]. PLCVC does not cause significant clinical signs, but rarely, it can be associated with megaesophagus [4]. Currently, surgical ligation and catheter-based techniques are available for treatment [5–8]. No specific breed or sex predilection has not been detected in dogs [9]. Generally, PLCVC coexists with other cardiac defects and is mostly detected on cardiovascular imaging performed for other purposes [10,11]. PDA can be diagnosed using physical examination, X-ray, and echocardiography, but computed tomography (CT) is required to detect the presence of other vascular malformations. The purpose of this case report was to describe the diagnosis and surgical treatment of PLCVC and PDA in a dog.
Case History
A 3-month-old, 4.9-kg, intact male Bichon Frise was incidentally detected with murmur on auscultation at a local animal hospital one week before. The dog was referred to the Animal Medical Center, Gyeongsang National University. Despite mechanical murmur and abnormal echocardiography, no significant clinical signs were present [12].
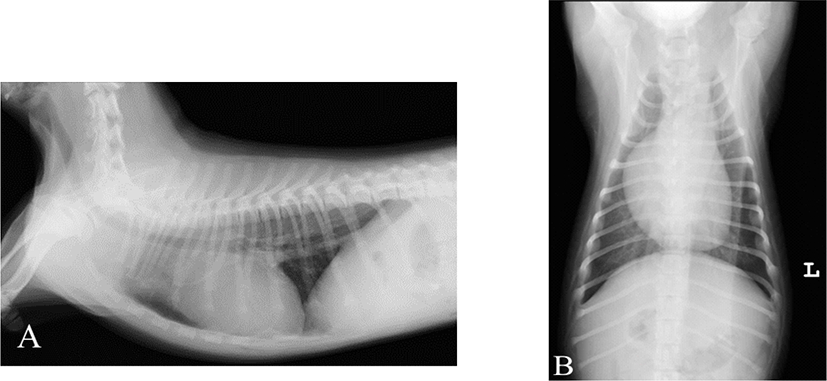
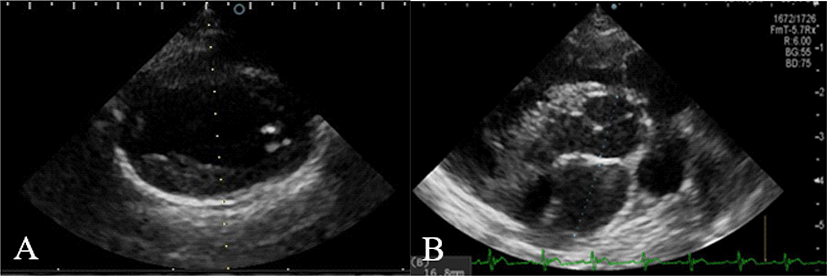
On physical examination, no significant findings other than continuous mechanical murmur were detected. Thoracic radiography and echocardiography were performed for the definitive diagnosis. Pulmonary vein dilation, right atrium bulging, right ventricular enlargement was observed on the chest X-ray (Fig. 1). On echocardiography, the mean pulmonary artery turbulent flow, dilation, and continuous ductal flow were observed (Fig. 2). Left ventricular and atrial dilation, flattening of the interventricular septum, paradoxical movement, and progressive myocardial deterioration were also observed. Moreover, mild pulmonary regurgitation and moderate right ventricular outflow tract velocity were observed. Due to the result, congenital disease was suspected. Therefore, PDA was diagnosed on imaging, and left thoracotomy was performed [13].
The dog was sedated with butorphanol (0.2 mg/kg, SC, Butophan®; Myungmoon Pharm, Seoul, Korea), diazepam (0.2 mg/kg, IV, Diazepam®; Sam-Jin, Seoul, Korea) and atropine (0.04 mg/kg, SC, Atropine®, Jeil Pharmaceutical Co., Daegu, Korea); anesthetized with etomidate (2 mg/kg, IV, Etomidate-®Lipuro, B. Braun Melsungen AG, Germany); and intubated. Cefazolin (25 mg/kg, IV, Hankook Korus Pharm Co., Seoul, Korea) was used as a prophylactic antibiotic. Anesthesia was maintained using a mixture of 2.0%–3.0% isoflurane (TerrellTM, Piramal Critical Care, USA) and oxygen. Heart rate was monitored, and respiratory rate was maintained between 8–20 breaths/min.
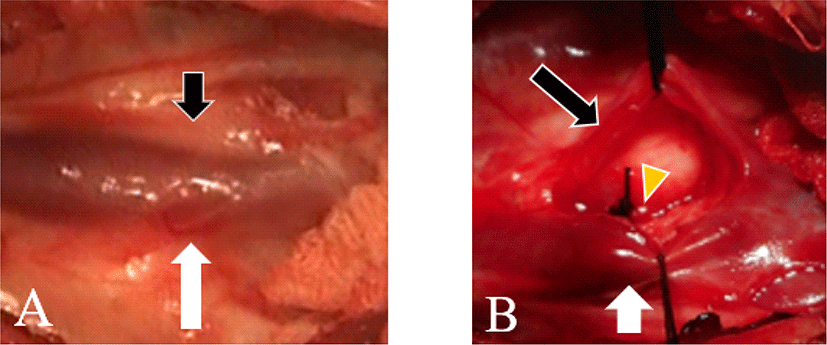
During surgery, the anatomical structure was found to be different from typical PDA (Fig. 3). PLCVC was positioned immediately above PDA. Therefore, the surgeon dissected PLCVC carefully and lifted it ventrally to obtain a clear surgical view of PDA. To ligate PDA, a black silk 4–0 was used. Post operatively, Carprofen (2.2 mg/kg, PO, twice daily [bid], Carprofen®, Zoetis, USA), cefazolin (25 mg/kg, IV, Hankook Korus Pharm Co., Seoul, Korea), butorphanol (0.2 mg/kg, SC, Butophan®; Myungmoon Pharm, Seoul, Korea) and famotidine (0.5 mg/kg, PO, bid, Famotidine®; Nelson, Korea) were used. The patient was followed up for six postoperative months with radiographic examination, which showed satisfactory prognosis.
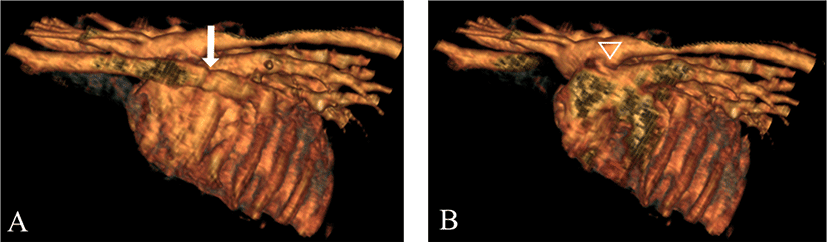
Chest X-ray, echocardiography, and CT angiography were performed to check the postoperative prognosis and any other vascular malformations. No significant changes were seen on X-ray. On echocardiography, a linear hyperechoic structure was identified with declined flow to the main pulmonary artery. On CT angiography, the non-atrophied left brachiocephalic vein retaining connection with the coronary sinus draining to the right atrium was observed, which was defined as PLCVC (Fig. 4).
Discussion
This case report presented the detection of PLCVC with the congenital heart defect PDA in dogs. Canine embryo development normally forms venous returns to the heart through the paired cranial and caudal cardinal veins, which pair to form the common cardinal veins. These veins allow blood to flow into the sinus venosus, which becomes the right atrium. Because of the direct fusion cranial to the heart, the right and left cranial cardinal veins are present. As the embryo develops, the remaining left cranial cardinal vein atrophies while the right-side vessel enlarges and becomes the distal part of the cranial vena cava. The right common cardinal vein forms the right cranial vena cava, and left cardinal system mostly atrophies, which forms the coronary sinus [13]. Myocardial venous blood flows to the coronary sinus, which drains into the right atrium [14].
PLVCV is rare in dogs, and it is mostly accompanied with congenital heart diseases, such as PDA and persistent right aortic arch (PRAA) [15]. Identification of PLCVC can be done on CT angiography and at the time of surgery [16]. Many dogs with other congenital heart problems suffer respiratory distress, and in severe cases, sudden death [17]. Congenital heart defects are life-threatening, resulting in euthanasia in several veterinary cases [10,18]. Therefore, surgery is commonly performed for other congenital heart diseases. However, PLCVC itself does not cause clinical problems which makes it difficult to detect without CT angiography in dogs without congenital heart diseases [19]. In this case, significant continuous mechanical murmur was detected in auscultation, and turbulent flow and continuous ductal flow was observed in echocardiography. But, PLCVC was not detected in echocardiography before the surgery.
During the surgery, it was difficult to find the landmarks of PDA. PLCVC was located immediately above the PDA and vagus nerve [20]. PLCVC made it harder to find the landmarks, vagus nerve and patent duct. As this case had no preoperative CT angiography, the surgery was more challenging and increased the time of surgery. In another case report, a dog with PDA and PLCVC was reported. They also describe that the PLCVC was detected and located in exactly upper side of the PDA. The difference between this case report and the one reported before is visualization of vagus nerve. Our case was unable to visualize the location of the vagus nerve because of PLCVC. Therefore, in order to find out the exact location of the landmarks of PDA beneath PLCVC, it is important to perform preoperative CT.
In conclusion, PLCVC was accompanied with the congenital heart disease PDA in a dog. In dogs that do not show clinical signs, PLCVC can be undetected or incidentally found [16]. The dog in this case had PDA with no significant clinical signs, and radiographic examination and surgery were performed. PLCVC can cause difficulties in the surgery [21]. If it is detected before the surgery, the surgery can be shorter and can provide better results. CT angiography is advantageous to visualize the location of PLCVC and variations in other thoracic veins in dogs [22].