Introduction
Capsule endoscopy is a non-invasive endoscopic imaging technique that has been used in human medicine for more than 10 years [1]. In addition, capsule endoscopy can directly asses the areas of the small intestine that cannot be seen through conventional gastrointestinal endoscopy [2–4]. Because of these advantages, it has been established as a major diagnostic method for gastrointestinal disease in human medicine [2, 3, 5]. It is commonly used in patients with obscure gastrointestinal bleeding, and to evaluate various gastrointestinal disorders such as Crohn's disease and tumors [2, 3, 5]. Recent studies have shown that the diagnostic value of capsule endoscopy is superior to other small intestine examination methods such as magnetic resonance imaging (MRI), computed tomography (CT), and intraoperative enteroscopy [6–8]. Consequently, capsule endoscopy has also been performed in veterinary clinical practice [1, 6–9].
Although capsule endoscopy is generally considered a safe procedure, capsule retention remains the most relevant procedure-related complication [4]. Capsule retention is that capsule endoscopy cannot complete imaging of the entire digestive tract in limited battery life. A retained capsule is usually asymptomatic, but can lead to partial or complete bowel obstruction [10, 11]. Symptomatic bowel obstruction may also require surgical or endoscopic removal of the retained capsule [11]. Studies have shown that, in human medicine, capsule endoscopes fail to reach the large intestine in about 20% of patients [12]. In veterinary medicine, there is a higher probability that the capsule endoscope will not pass through the pylorus [1, 9]. In other words, animals have higher capsule retention in their stomachs. The results of these studies in veterinary medicine showed that the pyloric passage of capsules endoscope was a very important factor in small animals of small intestine size compared with humans [9]. Therefore, in order to apply capsule endoscopy in veterinary medicine, it is necessary to develop capsules suitable for small animals [9]. The purpose of this study was to compare the difference in gastrointestinal transit time between a conventional capsule endoscope and a minimized capsule endoscope model in normal dogs to verify whether the minimization of capsule endoscopy can help relieve the capsule retention, especially the pyloric passage.
Materials and Methods
Three adult male Beagles weighing 9.0, 9.2, and 11.8 kg were used in this experiment. Their average weight was 10 kg. All dogs were similar in age, gender, breed, and body weight (Table 1). They were housed individually in cages and fed commercial dry food regularly. Physical examination, complete blood count, serum chemistry and X-ray were performed for all dogs before capsule endoscopy. No specific issues were identified. For 6 months prior to the experiment, all dogs had no history of drug use or specific digestive tract symptoms. Based on these results and their history, they were determined to be clinically healthy.
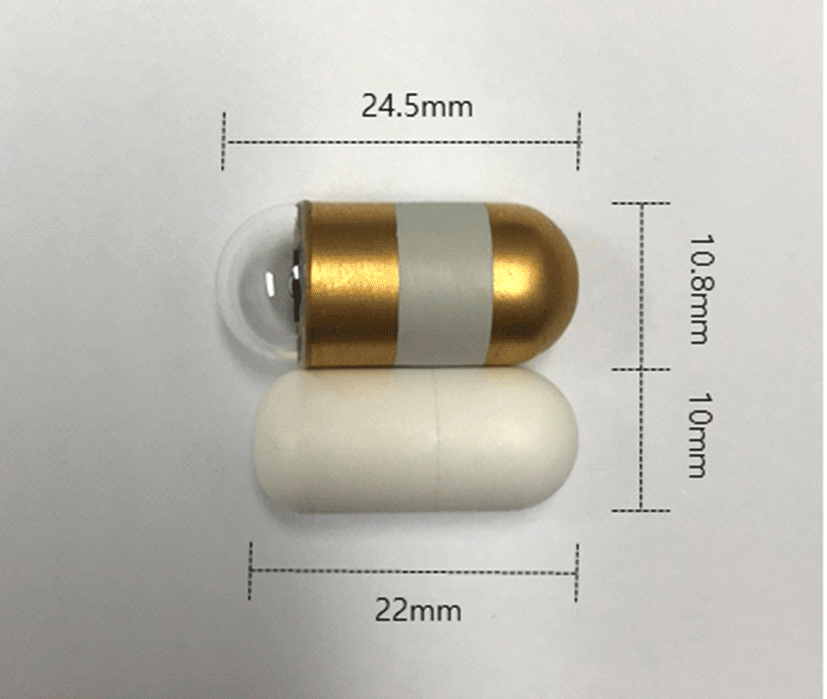
A conventional capsule endoscope and a minimized capsule endoscope model were used for the experiment. The conventional capsule endoscope is a MirocamⓇ (MC- 1200-M, Intromedic, Seoul, Korea) with a diameter of 10.8 mm and a length of 24.5 mm. It contained a small camera, flash, battery and transfer device. The minimized capsule endoscope model was manufactured by the same company and had a diameter of 10 mm and a length of 22 mm. This is the smallest size capsule that can be made using current technology, taking into consideration battery life, safety, visibility in the digestive tract, and cost (Fig. 1).
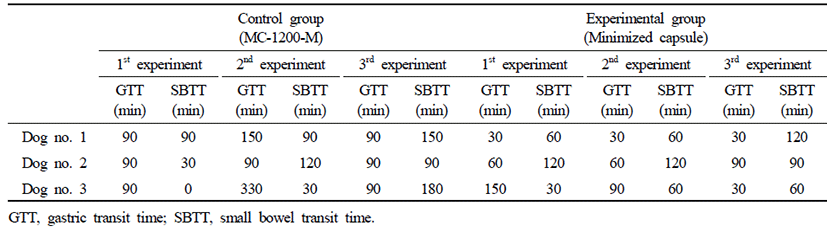
Three of the same Beagle dogs that participated in the experiment were classified into the control group which was orally administered the conventional capsule endoscope and the experimental group which was orally administered the minimized capsule endoscope model. Considering that the results may be different even in the same experiment on the same subject, experiments were performed 3 times for each dog. In other words, 9 experiments were performed per group.
Radiographs were taken immediately after swallowing of the capsules. They were performed every 30 minutes until the capsule reached the large intestine through the ileocolic valve.
All dogs were fasted for at least 12 hours before the examination. Each experiment was performed at the same time each day with more than 3 days interval considering the vital rhythm. The activity of all dogs were restricted in the cage during the experiment.
All dogs were treated in accordance with the guidelines approved by t3. Imaging Analysishe Institutional Animal Care and Use Committees (IACUC) of Gyeongsang National University (approval no. GNU-190409-D0020).
To analyze gastric transit time (GTT) and small bowel transit time (SBTT), radiographic machine (Regius model 190Ⓡ, KONICA, Tokyo, Japan) was used for taking the right lateral view and ventrodorsal view at intervals of 30 minutes in all dogs. These serial radiographic images were assessed on a DICOM workstation to determine the location of the stomach, small intestine, and large intestine in which the capsule was located, images were evaluated with consent of 4 radiologists.
Because it was difficult to evaluate the exact GTT and SBTT by radiography, the time at which the capsule was identified for the first time in the small intestine on the radiographic image was determined as GTT. From the time that GTT was identified, the time at which the capsule was first identified in the large intestine was determined as SBTT.
Results
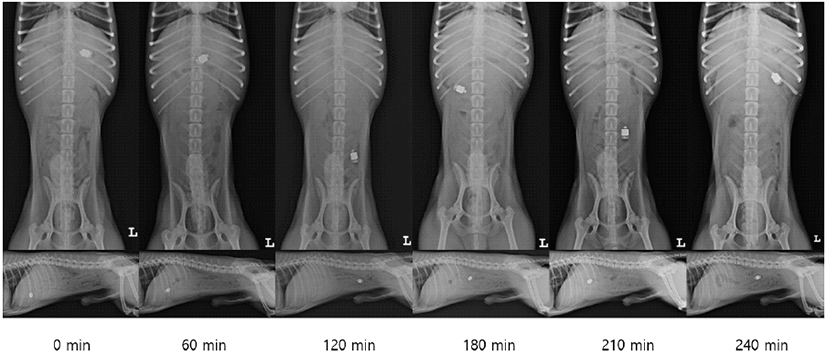
GTT and SBTT shown in the two groups are listed in Table 2. Serial radiographic images in this study are shown in Fig. 2. In both groups, the capsule passed through the entire digestive tract without any retention.
The control group with the oral administration of the conventional capsule endoscope represented average GTT of 123.3 minutes (SD ± 80 minutes), SBTT of 86.6 minutes (SD ± 58.9 minutes), and GITT of 210 minutes (SD ± 80.7 minutes). The experimental group with oral administration of minimized capsule endoscope model represented average GTT of 63.3 minutes (SD ± 40.9 minutes), SBTT of 80 minutes (SD ± 33.5 minutes), and GITT of 143 minutes (SD ± 41.8 minutes).
There was a significant difference in GTT between the control group using the conventional capsule endoscope and the experimental group using the minimized capsule endoscope model (control group: 123.3 ± 80 minutes when compared with experimental group: 63.3 ± 40.9 minutes, p=0.019) (Fig. 3A).
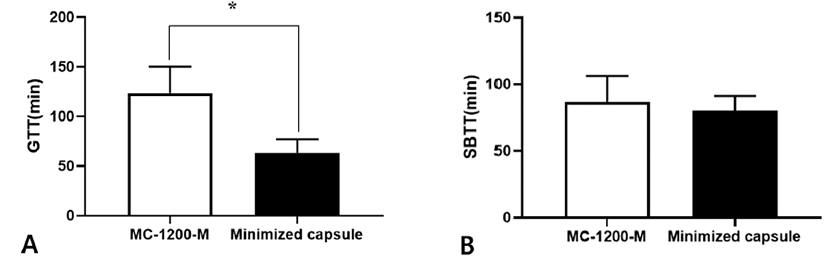
There was no significant difference between the control group using the conventional capsule endoscope and the experimental group using the minimized capsule endoscope model (control group: 86.6 ± 58.9 minutes when compared with experimental group: 80 ± 33.5 minutes, p=0.863) (Fig. 3B).
Discussion
Capsule endoscopy has many advantages including it is non-invasive, has no risk of anesthesia, and has the ability to directly assess all segments of the small intestine [1– 5]. However, capsule retention in the gastrointestinal tract remains the biggest problem not only in humans, but especially in small animals [1, 4, 9–13]. Capsule retention is when capsule endoscopy does not complete the imaging of the entire digestive tract in limited battery life. In human medicine, it has been defined as having a capsule retained in the gastrointestinal tract for a minimum of two weeks [14]. Several studies have shown that capsule retention is not related to the size of the capsule or the age of the patient. Moreover, it is known that the probability of occurrence varies depending on the type of underlying disease the patient has [15–22]. The highest probability of capsule retention occurred in patients with subacute small bowel obstruction (10%–20%) or in small bowel tumours (10%–25%) among patients undergoing capsule endoscopy, and 8% in patients with established inflammatory bowel disease (IBD) [23–27].
In veterinary medicine, there are a few studies related to capsule retention. In a study evaluating the gastrointestinal motility of capsule endoscopy using 23 beagle dogs, a capsule endoscope failed to pass through the pylorus at 27 of 40 attempts [9]. In another study conducted using 2 healthy dogs and 8 patients to detect mucosal lesions associated with gastrointestinal bleeding, retention was not observed in 2 healthy dogs, but capsule endoscope did not pass through the pylorus in 3 out of 8 patients [1]. In another study conducted with 18 beagle dogs to evaluate anthelmintic efficacy, capsule endoscope remained in the stomach until the battery had been exhausted in 3 out of 18 beagle dogs [13]. These results showed a higher rate of capsule retention, compared to humans where the incidence of capsule retention ranges from 0 to 21% [28 –30]. Unlike in humans, all three of the above studies showed that a capsule once passed through the pylorus does not show any retention in the small intestine [1, 9, 13]. This suggests that the passage of the capsule endoscope through the pylorus was a very important factor in small animals with a small diameter of the small intestine compared with humans. There is, therefore, a need to develop capsule endoscopes of appropriate size for small animals for clinical application in veterinary medicine [9]. Considering the difference between the weight of dogs used in the above 3 studies (7–40 kg) and the weight of domestic companion animals (mostly small dogs weighing less than 5 kg), it is important to develop a smaller capsule endoscope [1, 9, 13]. Capsule endoscopes, currently licensed and used for animals in the USA and Korea, are also recommended for use only in dogs weighing more than 6 kg, although they have succeeded in those weighing 4.5 kg. It is also impossible to apply in cats, that are suffering from small intestine diseases regardless of their weight.
The current study compared the GTT and SBTT between the control group which was orally administered the conventional capsule endoscope and the experimental group which was orally administered the minimized capsule endoscope model. We found that, the mean SBTT of the control group was 86.6 ± 58.9 minutes and the mean SBTT of the experimental group was 80 ± 33.5 minutes. Following capsule minimization, there was a slight decrease in SBTT, which was considered insignificant. Additionally, capsule retention in the small intestine was not observed, as in previous studies [1, 9, 13]. However, significant differences in GTT were observed between both groups following minimization of the capsule endoscope. The GTT averaged 123.3 ± 80 minutes in the control group and 63.3 ± 40.9 minutes in the experimental group.
These results are pertinent as they showed that minimization of capsule endoscope can relieve the capsule retention, which is a major complication of capsule endoscopes, especially the gastric congestion caused by the capsule's failure to pass through the pylorus in small animals, and increase the utilization of the capsule endoscopy in veterinary clinical practice. With the minimization of capsule endoscopes and the advancement of technology, future capsules should contain sensors for pH measurement, peristalsis and detecting cancer markers, and an endoscopic ultrasound probes must be installed [31]. Despite the technical and financial limitations in veterinary medicine, the development of capsule endoscopy will significantly improve the diagnosis of gastrointestinal disease [31].
There are several limitations to this study. First, there is a low number of research population. A study with a larger research population will be necessary to derive more meaningful results. Moreover, the minimized capsule endoscope model is still a model step. Although the model was created considering the various parts of the actual capsule endoscope, there will certainly be differences in the future development process. Finally, we used only an average of 10 kg beagle dogs. Considering that domestic companion animals are mostly small dogs weighing less than 5 kg, further studies on smaller animals are needed.