Original Article
Novel porcine model of acute myocardial infarction using polyethylene terephthalate
Yong Sook Kim1
,2
,†

, Nan Yeol Kim3
,†, In-Ho Bae1
,2
,4

, Jun Kyu Park5

, Dae Sung Park1
,2
,4
,6

, Jae Won Shim1
,2
,4

, Wan Suk Kang1
,2, Han Byul Kim1
,2

, Bong-Seok Song7

, Bon-Sang Koo7, Kang-Jin Jeong7, Sun-Uk Kim7

, Hyun Kuk Kim7

, Sung Soo Kim8

, Min Chul Kim8, Doo Sun Sim1
,2

, Young Joon Hong1
,2

, Youngkeun Ahn1
,2

, Kyung Seob Lim7
,*

, Myung Ho Jeong1
,2
,4
,*

1Cardiovascular Convergence Research Center of Chonnam National University Hospital Designated by Korea Ministry of Health and Welfare, Gwangju 61469, Korea
2Cardiovascular Research Center, Chonnam National University Hospital, Gwangju 61469, Korea
3Departments of Thoracic and Cardiovascular Surgery and Cardiology, Wonkwang University School of Medicine, Iksan 54538, Korea
4Korea Cardiovascular Stent Research Institute, Jangsung 57248, Korea
5CGBio Co. Ltd, Jangseong 57248, Korea
6Research Institute of Medical Sciences, Chonnam National University, Gwangju 61186, Korea
7Futuristic Animal Resource and Research Center & National Primate Research Center, Korea Research Institute of Bioscience and Biotechnology, Ochang 28116, Korea
8Department of Internal Medicine and Cardiovascular Center, Chosun University Hospital, University of Chosun College of Medicine, Gwangju 61453, Korea
These authors equally contributed to this work.
*Corresponding author: Kyung Seob Lim Futuristic Animal Resource and Research Center & National Primate Research Center & Korea Research Institute of Bioscience and Biotechnology, Cheongju 28116, Korea Tel: +82-43-240-6308, Fax: +82-43-240-6309, E-mail:
dvmlim96@kribb.re.kr Myung Ho Jeong Department of Internal Medicine and Cardiovascular Center, Chonnam National University Hospital, Gwangju 61469, Korea Tel: +82-62-220-6243, Fax: +82-62-228-7174, E-mail:
myungho@chollian.net,
mhjeong@chonnam.ac.kr
© Research Institute of Veterinary Medicine, Chungbuk National University. All rights reserved.
Received: Mar 12, 2019; Revised: Jun 19, 2019; Accepted: Jun 20, 2019
Abstract
Acute myocardial infarction (AMI) is considered the major cause of mortality in the world. Tremendous animal studies are performed to develop novel therapeutics, and this study aimed to induce porcine myocardial infarction model by using polyethylene terephthalate (PET). Coronary guidewire was placed in left anterior descending artery (LAD). The balloon angioplasty catheter was inserted at the back of the PET. The balloon catheter was carefully pushed forward, until the balloon marker was located in mid-LAD. Coronary angiography was performed pre- and post-occlusion at 28 days by C-arm. Histologic analysis of heart tissue was performed 28 days after inducing AMI. Thirty three pigs were anesthetized and underwent percutaneous coronary catheterization. All pigs were successfully embolized in mid-LAD by PET. Fifteen pigs died due to ventricular fibrillation during post-anesthetic recovery time, and overall experiment mortality was 45.5%. In 2,3,5-triphenyl tetrazolium chloride staining, gross finding of the ischemic heart lesion showed firm and white area of infarction associated with the apex and left ventricular posterior wall. Infarct on H&E-stained sections demonstrated a region without myocytes and rich with cardiomyocyte with atypical nuclei. Successful induction of AMI by using PET may provide the pathophysiological information of ischemic heart disease and improvement of therapy development for AMI.
Keywords: polyethylene terephthalate; porcine model; myocardial infarction; interventional cardiology; cardiovascular disease
Introduction
Coronary artery disease (CAD) and acute myocardial infarction (AMI) are major causes of mortality and morbidity in the world and are estimated to be responsible for 15%–20% of deaths in the United States [1]. To overcome and treat CAD, numerous AMI models using rodents, sheep, dogs, and pigs have been developed to study the associated pathophysiological, cellular, and molecular changes.
Among these, porcine models are preferred because of the similarity of the coronary artery anatomy and heart/body ratio to that of humans. Additionally, polyethylene terephthalate (PET) is widely used in the medical field due to its documented excellent biocompatibility. PET has been used to replace a diseased segment of artery. PET is a commercially available material with an excellent mechanical strength, good stability against body fluids, and high radiation resistance for sterilization [2, 3].
In this study, we utilized PET to occlude the left anterior descending coronary artery of pigs to develop a novel AMI model.
Materials and Methods
Animal preparation
This animal study was approved by the Ethics Committee of Chonnam National University Medical School and Chonnam National University Hospital (CNU IACUC-H- 2013-12), and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Our study included a total of 33 pigs from the animal catheterization laboratory of Chonnam National University Hospital. Yorkshire × Landrace F1 crossbred castrated male pigs (20–25 kg) were observed in the laboratory animal center of Chonnam National University Medical Institute for 5–10 days before the experiment. All pigs were housed under controlled environmental conditions and veterinarian care. Premedication with aspirin 100 mg and clopidogrel 75 mg per day was given for 5 days before the procedure [4–6].
Materials
Vascular repair material made of PET was obtained (DuPont Co. USA).
Preparation of polyethylene terephthalate occlude
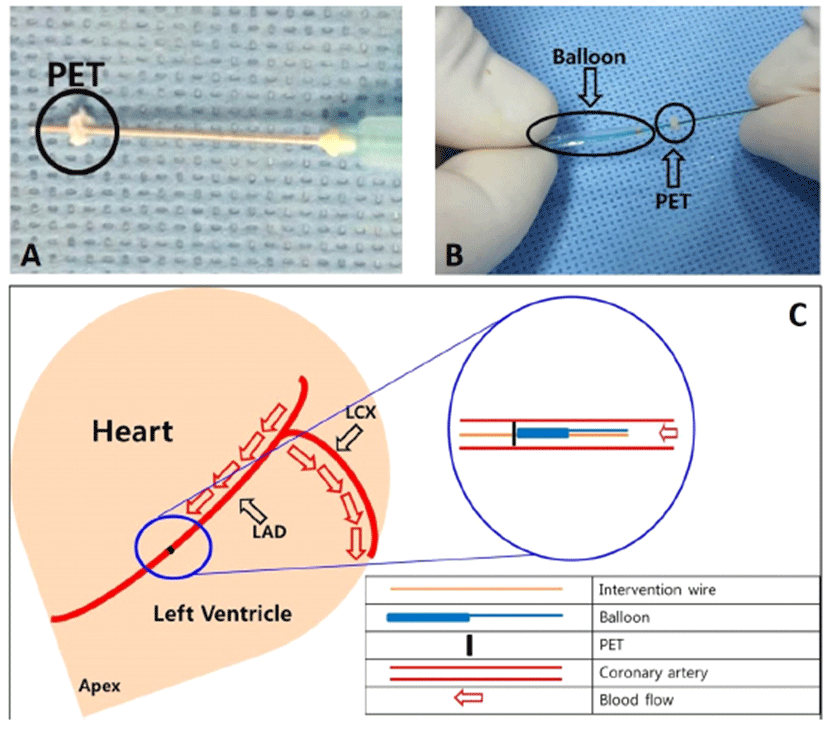
The PET occluder was manufactured with material trimmed from a woven polyester graft (Fig. 1A). The size of the PET occluder was adjusted to the size of the left anterior descending coronary artery.
Fig. 1.
Preparation of PET occlude (A). A representative image of the actual experiment. The balloon catheter was inserted at the back of the PET occlude (B). A schematic diagram of the experimental procedure (C). PET, polyethylene terephthalate; LAD, left anterior descending artery; LCX, left circumflex artery.
Download Original Figure
Anesthesia
All pigs were fasted for 24 h with unlimited access to water before the procedure. On the day of the procedure the pigs were anesthetized with zolazepam and tiletamine (2.5 mg/kg, Zoletil50®, Virvac, Caros, France), xylazine (3 mg/kg, Rompun®, Bayer AG, Leverkusen, Germany), and azaperone (6 mg/kg, Stresnil®, Janssen-Cilag, Neuss, Germany). An intravenous (IV) catheter was placed in the marginal ear vein for the administration of fluid and emergency drugs such as epinephrine and anti-arrhythmic agents (amiodarone hydrochloride). IV fluid administration with saline 0.9% was continued throughout the experiment. After intubation, anesthesia was maintained with inhalation anesthetic consisting of sevoflurane (1%) in oxygen (100%) [7]. Pigs were mechanically ventilated. Tramadol HCl (5 mg/kg, Trodon®, Aju pharm, Korea) was administered IV pre- and post-operatively for reducing pain.
Protocol for AMI induction
Lidocaine (2% solution) was administered subcutaneously at the left cut-down site. The left carotid artery was surgically exposed, and a 7F introducer sheath with a side port was placed in the left carotid artery.
A 7F coronary artery guiding catheter was placed within the opening of the coronary artery and a baseline coronary angiogram was obtained using the nonionic contrast agent iohexol (Omnihexol 300, Korea United Pharm Co., Seoul, Korea) under fluoroscopic guidance with a mobile fluoroscopy system (BV Pulsera, Philips Medical Systems, Andover, MA, USA).
A 0.014″ interventional guide wire with the attached PET occluder was advanced through the guiding catheter and placed across the left anterior descending artery (LAD). We used balloon catheters once inflation and then deflated to negative pressure using indeplator to prevent slipping of PET occluder from the interventional wire into the back of the balloon. A balloon angioplasty catheter was inserted into the mid-LAD until it was just proximal to the PET occluder and was carefully pushed forward until the balloon marker was located in the middle LAD and the occluder was lodged in the LAD (Fig. 1B and 1C). First remove the interventional wire and then remove the balloon. PET occlude is then placed in front maker portion of the removed balloon.
Angiography was performed to confirm obstruction of the distal-LAD. Finally, the guide wire, balloon catheter, and guiding catheter were removed and the left carotid artery was ligated.
Electrocardiographic monitoring for AMI and arrhythmia confirmation
Electrocardiography monitoring was performed throughout the experiment. Acute occlusion of the coronary artery was confirmed by changes in the normal ST segment at baseline and ST elevation during cardiac ischemia on continuous electrocardiography. All pigs were observed for at least 1 h for the development of cardiac arrhythmias such as ventricular fibrillation or tachycardia.
Evaluation of left ventricular systolic function by using two-dimensional transthoracic echocardiography
Transthoracic echocardiography (TTE) was performed prior to (baseline, before AMI), 1 hour after, and 4 weeks after AMI. All the surviving pigs were examined to assess the left ventricular ejection fraction (LVEF), which represents left ventricular systolic function. Cardiac images were taken by using an echocardiography system (Vivid S5, GE Healthcare, Schenectady, NY, USA) under general anesthesia while the pigs were in the supine position. Left ventricular end-systolic (left ventricular endsystolic volume; LVESV) and end-diastolic dimensions (left ventricular end-diastolic volume [LVEDV]) were determined from two-dimensional imaging, and LVEF was calculated by using the modified biplane method [8].
Pathological analysis of infarcted lesion
A follow-up coronary angiogram was performed 4 weeks post-AMI. At the end of the experiment, pigs were anesthetized sacrificed with an overdose of potassium chloride. The hearts were rapidly removed, extracted and grossly sectioned at 1-cm intervals. The myocardial sections were stained with 2,3,5-triphenyl tetrazolium chloride (TTC) solution (1% in phosphate-buffered saline) for 30 min at 37℃. After TTC staining, sectioned heart tissues were fixed in 10% neutral buffered formalin overnight, and embedded in paraffin for histological analysis. The tissue samples were cut into 3- to 5-μm thick sections and stained with hematoxylin and eosin (H&E) and Masson’s trichrome. TTC, H&E, and Masson’s trichrome stains were performed to evaluate the infarcted area of the ventricle. Histological analysis of the infarcted myocardium was performed by an experienced cardiac pathologist.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) for Windows, version 16.0 (IBM, Armonk, NY, USA). The data are presented as means ± S.D. The baseline, 1 hour after AMI and 4-week follow-up echocardiographic data were compared using the paired t-test. A p-value < 0.05 was considered significant.
Results
AMI induction using PET
A total of 33 pigs were anesthetized and underwent percutaneous coronary catheterization. The left and right carotid arteries were successfully catheterized without any side effects such as bleeding, arterial dissection, or shock. All pigs successfully underwent embolization of the middle LAD with PET. Fifteen pigs died due to ventricular fibrillation during the post-anesthetic recovery time. Ventricular fibrillation occurred within 40 min after AMI induction. The overall study mortality was 45.5% (dead 15/total 33 pigs). None of the pigs manifested clinical signs of surgical site infection during the subsequent 4 weeks.
Coronary angiographic findings
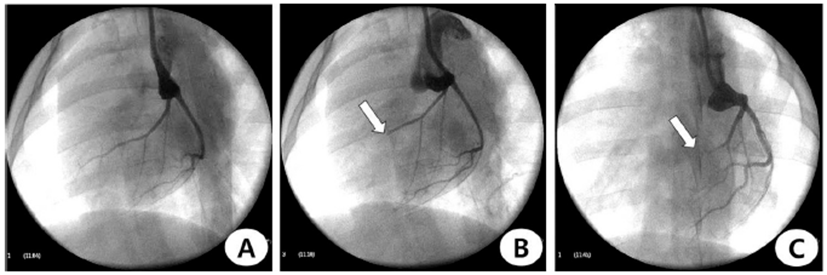
Left coronary angiogram confirmed occlusion of the LAD (Fig. 2A). After the procedure, repeated coronary angiogram showed acute obstruction of the distal LAD in this pig (Fig. 2B). The follow-up angiogram was performed 4 weeks post-AMI (Fig. 2C). All of LAD was occluded on follow-up coronary angiogram in 18 pigs.
Fig. 2.
Coronary angiograms during and after induction of anterior wall myocardial infarction. (A) baseline, (B) occlusion and (C) follow-up after 4 week of the distal-left anterior descending artery. White arrow indicates occlusion site.
Download Original Figure
Echocardiographic findings 1 hour and 4 weeks after AMI compared with baseline
Table 1 shows the echocardiographic data at baseline, and 1 hour after and 4 weeks before/after AMI. All the values changed after induction of myocardial infarction. Notably, the EF values were decreased by approximately 45% of the baseline after 1 hour of induction of AMI (59.4% ± 6.18% at baseline vs. 34.9% ± 6.45% at 1-hour follow-up; Table 1). On comparisons of echocardiography data among baseline, 1-hour and 4 weeks after AMI, LVEF (p<0.0001), LVEDV (p=0.0271) and LVESV (p= 0.0112) values decreased significantly from baseline to 1 hour after AMI. LVEF (p<0.0001) was decreased significantly, but LVEDV (p=0.4036) and LVESV (p=0.7726) was no differences between baseline and 4 week followup estimated value.
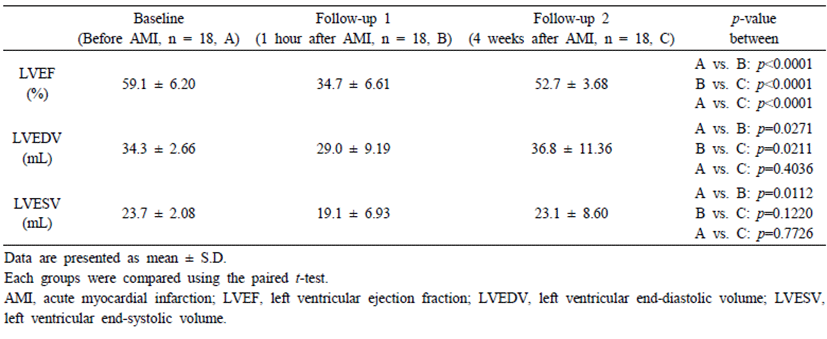
Table 1.
Echocardiographic data at baseline, and 1 hour and 4 weeks after acute myocardial infarction in the surviving pig
Gross and histological findings
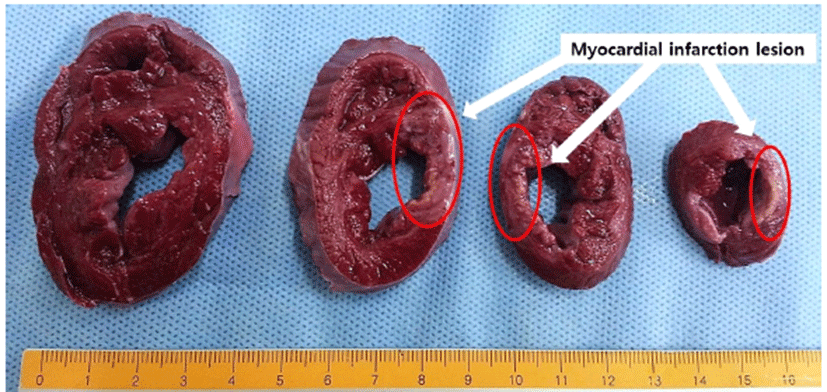
TTC staining grossly showed a firm white area of infarction involving the cardiac apex and left ventricular posterior wall. The white infarcted myocardium contrasted with the red color of the surrounding normal cardiac muscle (Fig. 3).
Fig. 3.
TTC staining at 5 weeks showing areas of infarcted myocardium in the left anterior ventricle wall (white arrows). TTC, 2,3,5, triphenyl tetrazolium chloride.
Download Original Figure
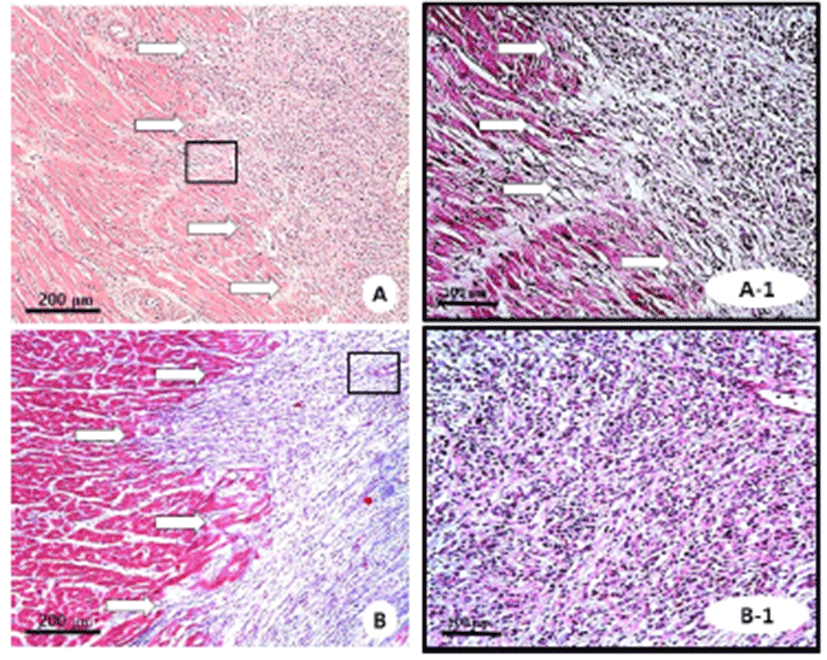
H&E-stained sections demonstrated a region without myocytes and rich with cardiomyocytes with atypical nuclei (Fig. 4A). Masson’s trichrome staining showed fibrosis and collagen deposition in the infarcted area (Fig. 4B).
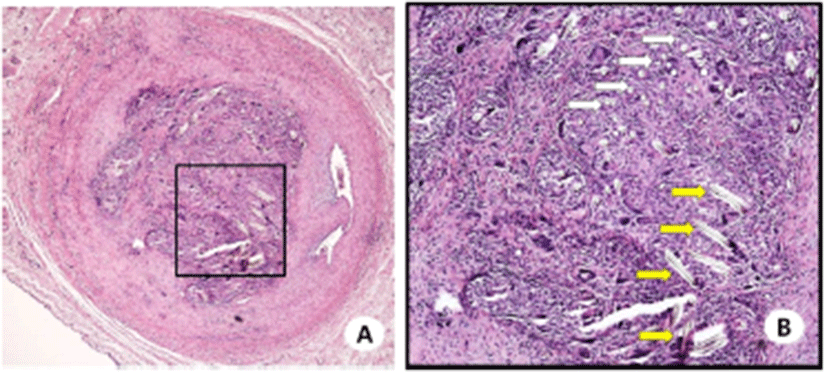
Histological examination of the PET occluder insertion site showed complete occlusion of the coronary artery. The fiber of PET occluder was observed in the occluded lesion (Fig. 5).
Fig. 4.
Hematoxylin and eosin (A, ×100; A-1, ×200) and Masson’s trichrome (B, ×100; B-1, ×200) staining of representative images in infarcted tissue. White arrows indicated infarcted tissue.
Download Original Figure
Fig. 5.
Hematoxylin-eosin-stained tissue sections of the coronary artery occlusion site (A, original magnification ×40) by the PET occluder (B, original magnification ×100; white arrow, short axis of the PET fiber, long axis of the PET fiber). PET, polyethylene terephthalate.
Download Original Figure
Discussion
This study was conducted to establish the novel method to induce an AMI using PET in the LAD of a pig, and demonstrates the successful induction of an AMI similar to that of a human. Our porcine AMI model may provide additional pathophysiological information regarding ischemic heart disease and allow the development of advanced cardiac therapeutics.
Despite improving patient survival rates, CAD, including AMI, remains a leading cause of cardiovascular mortality and morbidity in the world [9, 10]. Over the past few years, preclinical study by using animal models has become important for translational research. It is clinically important to verify cardiac therapeutics in large animal models prior to clinical applications. Pigs are considered as the most suitable animal for myocardial infarction model due to several advantages. The heart size is compatible with the human heart and cardiovascular function and hemodynamic parameters are also similar [11].
PET was chosen for this study because of its availability and widespread use in vascular surgery and interventional cardiology devices such as angioplasty balloon catheters and artificial blood vessels. Additionally, the biocompatibility of PET has been extensively studied [12– 14].
The present MI model has three advantages over balloon angioplasty occlusion or coronary coil deployment. First, the three methods are closed-chest approaches and considered noninvasive. Second, the main difference from balloon angioplasty occlusion is that the present model is a permanent occlusion. Last, unlike the PET occlusion method, the commercial embolization coil (VortX-18 Diamond 3 mm/3.3 mm coil, Boston Scientific/Target) is expensive.
The ischemia-reperfusion model using balloon catheter would be more close to the clinical situation of human patients with AMI. However, the permanent ischemia and ischemia-reperfusion models have different heart injury mechanism [15]. The lesion induction mechanism of our model is the blockage the coronary blood flow through an artificial scaffold (PET).
In addition, the permanent model has the advantage of confirming the lesion clearly compared to the reperfusion model. It would be better to use each model as a tool for the purpose of experiment.
Several animal models have been developed to evaluate novel drugs and devices, to study the pathophysiological changes of AMI, and to decrease mortality and subsequent heart failure.
The main difference among animal myocardial infarction models is use of invasive surgical approaches, or, as in the present study, non-invasive interventional catheter- based approaches for infarction induction.
AMI models have several differences between small (mouse and rat) and large animals (dogs, sheep, and pigs). The application of rodent AMI models is simpler and less expensive than large animal models. However, the coronary anatomy and pathophysiology of these animals are not similar to those of humans, and their small size precludes the testing of medical devices.
Sheep and pigs have been widely used as the large animal models of AMI. Tyler et al. created a sheep AMI model by using autologous-aggregated platelets, and other induction methods have been reported [16–19]. However, the left coronary artery of sheep demonstrates anatomical variation [20].
Researchers have preferred the pig model for cardiovascular research because of its greater similarity to the human coronary artery anatomy, clotting cascade, and cardiac pathophysiology. Furthermore, the heart-to-body ratio of the pig is similar to that of humans [21]. In addition, the size of conventional crossbred pigs weighing > 20 kg allows preclinical studies with coronary stents, intra-cardiac devices, artificial valves, and vascular grafts that may be eventually utilized in human patients.
As a result, most animal models have been replaced with porcine models when investigating cardiovascular devices and minimally invasive cardiac interventions.
Previous studies have used open chest surgery (amyloid constructor and surgical ligation) and cardiac intervention procedure (ethanol infusion, coil embolization, and balloon occlusion) to induce a porcine AMI [7, 21–34]. Our new model used interventional devices that are readily available and frequently used in clinical settings, and allows a simple and direct method to induce an ischemic lesion in the left ventricular myocardium without the need for non-biocompatible beads or invasive thoracotomy with coronary ligation [35, 36].
Results of stem cell experiment using this model were published [37]. Intramyocardial stem cell injections were performed around the borderline (infarcted and non-infarcted tissues). The reperfusion model using an angioplasty balloon did not present a clear boundary between these tissues. We are therefore developing and using this model.
Study limitations
A limitation of this study was that we did not evaluate the infarcted lesion and cardiac function with imaging modalities such as magnetic resonance imaging, technetium single photon emission tomography, or computed tomography.
Conclusion
We successfully induced an AMI in the territory of the LAD of pigs using a safe and feasible biomaterial PET. This model may provide further pathophysiological understanding of ischemic heart disease and be applied for therapeutic development for cardiac repair.
Acknowledgments
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2017R1C1B 1001956 and NRF-2018R1D1A1B07040783), and the KRIBB (Korea Research Institute of Bioscience and Bio technology) Research Initiative Program (KGM4251824), Korea.
References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:399-410.


Wu G, Tucker PA, Cuculo JA. High performance PET fibre properties achieved at high speed using a combination of threadline modification and traditional post treatment. Polymer 1997;38:1091-1100.

Ronkay F, Czigany T. Development of composites with recycled PET matrix. Polym Adv Technol 2006;17:830-834.

Alt E, Pinkernell K, Scharlau M, Coleman M, Fotuhi P, Nabzdyk C, Matthias N, Gehmert S, Song YH. Effect of freshly isolated autologous tissue resident stromal cells on cardiac function and perfusion following acute myocardial infarction. Int J Cardiol 2010;144:26-35.


Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J 2007;28:2667-2677.


Bai X, Yan Y, Song YH, Seidensticker M, Rabinovich B, Metzele R, Bankson JA, Vykoukal D, Alt E. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J 2010;31:489-501.


Regueiro-Purrinos M, Fernandez-Vazquez F, de Prado AP, Altonaga JR, Cuellas-Ramon C, Ajenjo-Silverio JM, Orden A, Gonzalo-Orden JM. Ventricular arrhythmias and mortality associated with isoflurane and sevoflurane in a porcine model of myocardial infarction. J Am Assoc Lab Anim Sci 2011; 50:73-78.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18: 1440-1463.


Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015;131:e29-322.

Fraccarollo D, Galuppo P, Bauersachs J. Novel therapeutic approaches to post-infarction remodelling. Cardiovasc Res 2012;94:293-303.


Appel JZ, Buhler L, Cooper DK. The pig as a source of cardiac xenografts. J Card Surg 2001;16:345-356.


Jiang Y, Liang Y, Zhang H, Zhang W, Tu S. Preparation and biocompatibility of grafted functional beta-cyclodextrin copolymers from the surface of PET films. Mater Sci Eng C Mater Biol Appl 2014;41:1-7.


Seitz H, Marlovits S, Schwendenwein I, Vecsei V, Losert U. [Biocompatibility of polyethylene terephthalate–PET– (Trevira strong)–an in vivo study of the sheep knee]. Biomed Tech (Berl) 1996;41:178-182.

Ramires PA, Mirenghi L, Romano AR, Palumbo F, Nicolardi G. Plasma-treated PET surfaces improve the biocompatibility of human endothelial cells. J Biomed Mater Res 2000;51:535-539.

Locatelli P, Olea FD, Hnatiuk A, De Lorenzi A, Cerda M, Gimenez CS, Sepulveda D, Laguens R, Crottogini A. Mesenchymal stromal cells overexpressing vascular endothelial growth factor in ovine myocardial infarction. Gene Ther 2015;22:449-457.


Betensky BP, Jauregui M, Campos B, Michele J, Marchlinski FE, Oley L, Wylie B, Robinson D, Gerstenfeld EP. Use of a novel endoscopic catheter for direct visualization and ablation in an ovine model of chronic myocardial infarction. Circulation 2012;126:2065-2072.


Charles CJ, Rogers SJ, Donald RA, Ikram H, Prickett T, Richards AM. Hypothalamo-pituitary-adrenal axis response to coronary artery embolization: an ovine model of acute myocardial infarction. J Endocrinol 1997;152:489-493.


Pilla JJ, Blom AS, Brockman DJ, Bowen F, Yuan Q, Giammarco J, Ferrari VA, Gorman JH, Gorman RC, Acker MA. Ventricular constraint using the acorn cardiac support device reduces myocardial akinetic area in an ovine model of acute infarction. Circulation 2002;106:I207-I211.
Hughes GC, Post MJ, Simons M, Annex BH. Translational physiology: porcine models of human coronary artery disease: implications for preclinical trials of therapeutic angiogenesis. J Appl Physiol 2003;94:1689-1701.


Jiang Y, Chang P, Pei Y, Li B, Liu Y, Zhang Z, Yu J, Zhu D, Liu X. Intramyocardial injection of hypoxia-preconditioned adipose-derived stromal cells treats acute myocardial infarction: an in vivo study in swine. Cell Tissue Res 2014;358:417-432.


Lee KH, Kwon SJ, Woo JS, Lee GJ, Lee SR, Jang HH, Kim HS, Kim JW, Park HK, Cho KS, Kim W. Effects of sildenafil on nanostructural and nanomechanical changes in mitochondria in an ischaemia-reperfusion rat model. Clin Exp Pharmacol Physiol 2014;41:763-768.


Chen CH, Chang MY, Wang SS, Hsieh PC. Injection of autologous bone marrow cells in hyaluronan hydrogel improves cardiac performance after infarction in pigs. Am J Physiol Heart Circ Physiol 2014;306:H1078-H1086.


Huang Z, Ge J, Sun A, Wang Y, Zhang S, Cui J, Zhang S, Qian J, Zou Y. Ligating LAD with its whole length rather than diagonal branches as coordinates is more advisable in establishing stable myocardial infarction model of swine. Exp Anim 2010;59:431-439.


Kim BO, Tian H, Prasongsukarn K, Wu J, Angoulvant D, Wnendt S, Muhs A, Spitkovsky D, Li RK. Cell transplantation improves ventricular function after a myocardial infarction: a preclinical study of human unrestricted somatic stem cells in a porcine model. Circulation 2005;112:I96-I104.
Biondi-Zoccai G, De Falco E, Peruzzi M, Cavarretta E, Mancone M, Leoni O, Caristo ME, Lotrionte M, Marullo AG, Amodeo A, Pacini L, Calogero A, Petrozza V, Chimenti I, D'Ascenzo F, Frati G. A novel closed-chest porcine model of chronic ischemic heart failure suitable for experimental research in cardiovascular disease. Biomed Res Int 2013;2013:410631.



Jun Hong S, Rogers PI, Kihlken J, Warfel J, Bull C, Deuter-Reinhard M, Feng D, Xie J, Kyle A, Merfeld-Clauss S, Johnstone BH, Traktuev DO, Chen PS, Lindner JR, March KL. Intravenous xenogeneic transplantation of human adipose-derived stem cells improves left ventricular function and microvascular integrity in swine myocardial infarction model. Catheter Cardiovasc Interv 2015;86:E38-E48.



Vilahur G, Juan-Babot O, Pena E, Onate B, Casani L, Badimon L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J Mol Cell Cardiol 2011;50:522-533.


Kim W, Jeong MH, Sim DS, Hong YJ, Song HC, Park JT, Ahn YK. A porcine model of ischemic heart failure produced by intracoronary injection of ethyl alcohol. Heart Vessels 2011;26:342-348.


Sim DS, Jeong MH, Kim YH, Choi S, Lim KS, Kim JH, Cho KH, Kim MC, Kim HK, Kim SS, Lee MG, Park KH, Hong YJ, Kim JH, Ahn Y, Cho JG, Park JC, Kang JC. Effects of sildenafil in combination with angiotensin-converting enzyme inhibitor on limiting infarct expansion in a porcine model of acute myocardial infarction. Int J Cardiol 2011;146:459-460.


Sim DS, Jeong MH, Song HC, Kim J, Chong A, Bom HS, Jeong IS, Oh SG, Kim JM, Park DS, Kim JH, Lim KS, Kim MS, Ryu SH, Kim HK, Kim SS, Jang SY, Cho JY, Jeong HC, Lee KH, Park KH, Yoon NS, Yoon HJ, Kim KH, Hong YJ, Park HW, Kim JH, Ahn Y, Cho JG, Park JC, Kang JC. Cardioprotective effect of fimasartan, a new angiotensin receptor blocker, in a porcine model of acute myocardial infarction. J Korean Med Sci 2015;30:34-43.



Sim DS, Jeong MH, Cha KR, Park SH, Park JO, Shin YM, Shin H, Hong YJ, Ahn Y, Schwartz RS, Kang JC. A reliable porcine coronary model of chronic total occlusion using copper wire stents and bioabsorbable levo-polylactic acid polymer. J Cardiol 2012;60:443-447.


Monnet E, Chachques JC. Animal models of heart failure: what is new? Ann Thorac Surg 2005;79:1445-1453.


Klocke R, Tian W, Kuhlmann MT, Nikol S. Surgical animal models of heart failure related to coronary heart disease. Cardiovasc Res 2007;74:29-38.