Introduction
Generally, conventional endoscopy technique used to examine small intestine has the limitation due to the length of small intestine, making it impossible to observe the overall area. In addition, it is an invasive examination that requires anesthesia [1]. Balloon endoscopy recently used to operate a dog has its strength in that it makes it possible to have a complete examination of the small intestine [2]. However, it can be only performed for large dogs. In addition, it involves intubation to the mouth and anus. In reality, it is hard to be performed commonly in veterinary medicine [2]. After the approval of capsule endoscope by the Food and Drug Administration of United States of America in 2001, it is now possible to safely observe the complete small intestine through a non-invasive method [3]. Since previous examination methods of small intestine are invasive with low sensitivity while the capsule endoscopy can conveniently and accurately observe the small intestine without risk of serious complications, capsule endoscopy is evaluated as the most appropriate method that is used widely now [1, 3]. Recently, it has been established as a major diagnosis method in human medicine for gastrointestinal disease. It is universally used in patients with obscure gastrointestinal bleeding and various digestive disorders such as Crohn’s disease, polyp, and tumor [1, 3–6]. Amongst various small intestine examination methods such as magnetic resonance enterography, computed tomography, and enteroscopy that have been research intensively in human medicine, the superiority of capsule endoscopy has been verified regarding its diagnostic value. Due to its convenience and superiority, there are attempts of performing capsule endoscopic examination in veterinary clinical trials [1, 4–8].
On the other hand, for complete assessment of the digestive system, the capsule must pass the total length of small intestine and reach the large intestine before its limited battery life runs out. In human medicine, around 20% of patients fail to meet the criteria to reach the large intestine in time. Depending on patients, there are cases that require surgery because the capsule is not discharged due to capsule retention [6]. In veterinary medicine, higher probability of such failure has been reported [7, 8]. Effect of gastrointestinal disease on the passing of capsule endoscope is currently unclear. However, in human medicine, it has been reported that underlying symptoms such as Crohn’s disease, diabetes, and tumor can act as factors that increase the likelihood of capsule retention in the gastrointestinal tract [6]. In human medicine, there has been many researches to overcome capsule retention, primarily focusing on the use of prokinetics [9]. In earlier capsule-related researches carried out in veterinary medicine considering such problem, the focus was set on capsule endoscope’s gastrointestinal transit time (GITT) and motility assessment [7, 8]. However, significant effect of prokinetics on capsule transit time has not been reported [7, 8]. Additional researches on its effects are needed. Thus, the objective of this prospective controlled study was to apply high doses of metoclopramide and mirtazapine to four healthy beagle dogs to assess their effects on transit time for capsule endoscopy.
Materials and Methods
Four adult male beagles weighing 9.0 to 11.8 kg were used in this experiment. All dogs had similar age and body weight. They were housed individually in cages and fed commercial dry food regularly. Physical examination, complete blood count, serum chemistry, and X-ray were performed for all dogs before capsule endoscopy. No specific issues were identified. For three months prior to the experiment, all dogs had no history of drug use or specific digestive tract symptoms. Based on these results and history, they were determined to be clinically healthy.
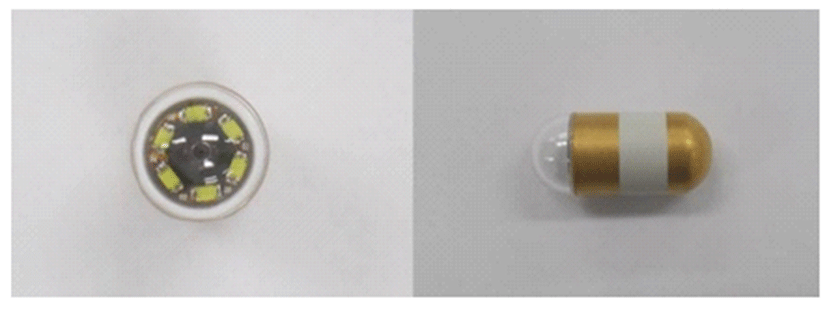
All dogs were fasted for at least 12 hours before examination. No bowel preparation was prescribed. Four beagle dogs participated in the experiment as two control groups, metoclopramide administered group and mirtazapine administered group at intervals of more than three days. Each dog was tested at the same time of the day considering circadian rhythm of the gastrointestinal tract. The capsule endoscope used in this experiment was a MiroCam® (MC1200-M, IntroMedic, Seoul, South Korea) with a diameter of 10.8 mm and a length of 25.5 mm. It contained a small camera, a flash, and a transfer device. This capsule endoscope can be used for a relatively longer time (about 12 hours) compared to conventional capsule endoscope (Fig. 1).
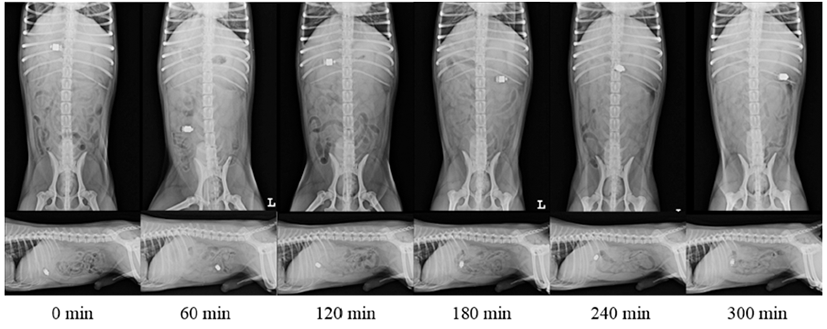
Two control groups received only capsules. In metoclopramide administered group, a high dose of metoclopramide (MeckoolⓇ Inj., Jeil Pharma., Daegu, South Korea) was administered as an experimental group. Metoclopramide was slowly administered as a bolus injection (1 mg/kg) from 15 minutes before swallowing capsule followed by continuous infusion at a rate of 1 mg/kg/h for the duration of the study period. In mirtazapine administered group, as another experimental group, mirtazapine (RemeronlⓇ, MSD-Korea, Seoul, South Korea) was orally administered at a dose of 15 mg/dog three hours before the experiment considering the time for mirtazapine to reach plasma peak level. Radiographs were taken immediately after swallowing of these capsules. They were performed every 30 minutes until the capsule reached the large intestine through ileocolic valve. Serial radiographic images taken in this study are shown in Fig. 2. Activity of all dogs was restricted in the cage during the experiment.
All dogs were treated in accordance with the guidelines approved by the Institutional Animal Care and Use Committees (IACUC) of Gyeongsang National University (approval no. GNU-190409-D0021).
To analyze gastric transit time (GTT) and small bowel transit time (SBTT), radiographic machine (Regius model 190®, KONICA, Japan) was used for taking the right lateral view and ventrodorsal view at intervals of 30 minutes in four beagle dogs. These serial radiographic images were assessed on a DICOM workstation to determine the location of capsule endoscope within the gastrointestinal tract. For accurate identification of the stomach, small intestine, and large intestine in which the capsule was located, images were evaluated with consent of four radiologists. In this experiment, because it was difficult to evaluate the exact GTT and SBTT by radiography, the time at which the capsule was identified for the first time in the small intestine on radiographic image was determined as GTT. The time at which the capsule was first identified in the large intestine from the time that GTT was identified was determined as SBTT.
Results
Basic characteristics of experiment dogs used in this study are shown in Table 1. This study was conducted with 4 beagle dogs with the same age of 25 months. Their average weight was 9.9 (range: 9–11.8 kg). They showed no notable difference in breed, age, gender, or weight (Table 1).
GTT, SBTT, and GITT found for the four groups are listed in Table 2. GITT of capsule endoscopy examination was 260 ± 100 minutes. In this study, all dogs of each group had successful passing of total gastrointestinal tract before the expiry of battery life. There was no capsule retention in this study.
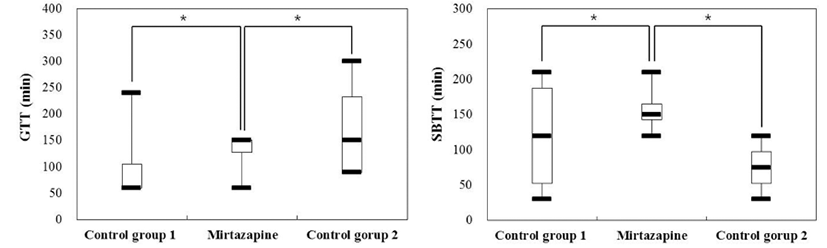
GTT was 105 ± 90 minutes and SBTT was 120 ± 88 minutes for control group 1 in which only capsule endoscopy was administered. GITT was shown to be 225 ± 151 minutes. For control group 2 that only endoscopy capsule was administered, it showed GTT of 173 ± 102 minutes, average SBTT of 75 ± 39 minutes, and GITT of 247 ± 115 minutes. For metoclopramide administered group where metoclopramide was administered by intravascular injection (1 mg/kg) followed by continuous infusion (1 mg/kg/h), it showed GTT of 248 ± 93 minutes, SBTT of 37.5 ± 15 minutes, and average GITT of 285 ± 99 minutes. For mirtazapine administered group in which mirtazapine was orally taken (15 mg/dog), it showed GTT of 128 ± 45 minutes, SBTT of 158 ± 38 minutes, and GITT of 285 ± 17 minutes. GTT and SBTT showed substantial differences depending on individual dog.
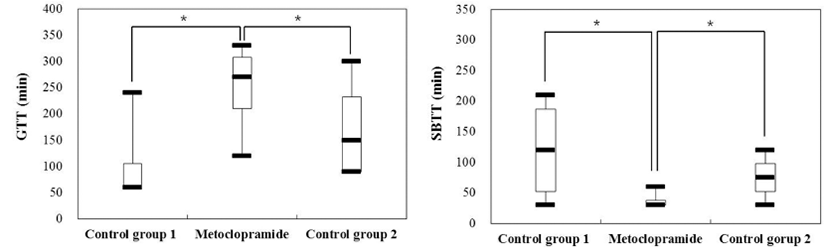
There was no significant difference in GTT for capsule only administered groups (control group 1 & 2) compared to metoclopramide administered group ([min] control group 1: 105 ± 90, control group 2: 172.5 ± 102 vs metoclopramide administered group: 247.5 ± 93, p = 0.07, 0.10). For SBTT, it showed a tendency of decrease ([min] control group 1: 120 ± 88, control group 2: 75 ± 39 vs metoclopramide administered group: 37.5 ± 15, p = 0.20, 0.18) in the metoclopramide administered group. However, such decrease was not statistically significant (Fig. 3).
There was no significant difference in GTT ([min] control group 1: 105 ± 90, control group 2: 172.5 ± 102 vs mirtazapine administered group: 127.5 ± 45, p = 0.56, 0.36) or SBTT ([min] control group 1: 120 ± 88, control group 2: 75 ± 39 vs mirtazapine administered group: 157.5 ± 38, p = 0.29, 0.07) between control groups 1 & 2 and mirtazapine administered group (Fig. 4).
Discussion
This study was conducted based on previous research results of both drug’s positive enhancement effect on rapid gastric emptying [10, 11]. However, metoclopramide or mirtazapine showed no significant effect on gastrointestinal movement of the capsule endoscope. Metoclopramide used as an experiment drug in this study has antiemetic and prokinetic effects on both the central nervous system and the gut [12, 13]. Considering that it is a drug that is most frequently used to treat dyspepsia and nausea with significant research results on human medicine’s capsule endoscopy and its wide use in veterinary clinical trials, it is used in this study [7, 9, 13]. It has been reported that metoclopramide can significantly enhance the ability of gastric emptying in both human and dogs by promoting contraction of antrum and pylorus and relaxation of the duodenum [10]. Unlike results shown in human medicine, the present research showed no significant enhancement of gastric emptying or small intestine motility in capsule endoscopy of dogs using metoclopramide. Since factors that determine gastric emptying of solid meals in dogs not only including organized movement of gastrointestinal tract, but also include secondary occurring factors that work different from humans in a complicated way such as the size of the object, composition, weight, and density, it is difficult to know clearly on which factor or factors might be the major contributor to the lack of influence of metoclopramide on the capsule’s gastrointestinal movement in dogs [14]. Various researches have shown that metoclopramide in solid form has less prokinetic effect than its liquid form [14, 15, 16]. A previous study on the effect of solid’s gastric emptying on dogs using radiolabeled technique to evaluate prokinetic effects of metoclopramide has shown similar results to the present study [17]. Various researches have also shown that metoclopramide for capsule endoscopy examination has no significant prokinetic effect [7, 8]. Metoclopramide’s half-life is known to be 90 minutes shorter in dogs compared to that in humans [12]. Thus, unlike previous studies, high dosage of metoclopramide (bolus loading dose of 1.0 mg/kg, IV, followed by continuous infusion at a rate of 1.0 mg/kg/h) was applied. However, it still had no statically significant results, confirming previous research results that used metoclopramide [7, 8]. Metoclopramide can resolve gastric disorder to already occurred retention of solid diet and ease its symptoms [15]. It is known to make the passing of food to pyloric easier by relaxing related areas, making it seem clear that metoclopramide is involved in systematical gastrointestinal movement control [10, 15]. It has been already reported that the effect metoclopramide on emptying of the sold phase is inferior to that on emptying of the liquid phase [16]. Additionally, metoclopramide has no effects on normal gastric emptying capabilities [15]. However, it is effective for patients with autonomic disturbance, especially effective on gastric emptying delay caused by dysfunction of vagus nerves for unclear reasons [15]. Researches that evaluated effects of prokinetics on capsule endoscopy in human medicine had practical patients as target subjects while researches from veterinary medicine used healthy dogs as target subjects [7, 16, 18, 19]. Additionally, it has been found that metoclopramide has minor effect on antrum, pylorus, and duodenum compared to other prokinetics such as cisapride [20]. Considering these results, a follow up research is required to find appropriate prokinetics for dogs.
Another controlled drug used in this study was mirtazapine. It is a noradrenergic and a specific serotonin antagonist. It is known to be an antidepressant with raised serotonin efficiency by blocking α2 adrenergic receptor to rapidly secrete serotonin and noradrenaline [11]. In recent years, mirtazapine’s prokinetic effects for treating functional dyspepsia suffering dogs showing no response to the conventional prokinetics have been proven [11].
Studies on effect of mirtazapine on rats have shown that noradrenaline plays a major role in intestinal functions by increasing the release of serotonin to control intestinal visceral sensation and acting as a primary neurotransmitter on parasympathetic nerve and central nervous system [21]. A research additionally conducted on dogs based on such results has shown that serotonergic 5-HT2 subtypes, especially subtype 5-HT2C that can stimulate the jejunum’s major contracting phase (phase III) and subtype 5-HT1A, can stimulate the large intestine’s migrating motor complexes to have effect on gastric emptying ability and large intestine movement ability [11]. Interestingly, mirtazapine, unlike previous prokinetics, does not affect the motility of small intestines with unclear reason [11]. However, in this study, similar to metoclopramide, mirtazapine failed to accelerate capsule movement within the gastrointestinal tract. The effect of mirtazapine on healthy dogs and patients with gastrointestinal disorder having gastric accommodation and visceral hypersensitivity damage who show no response to conventional prokinetics has been proven [11]. Considering the mechanism and research results of this drug, other secondary factors such as capsule’s size, composition, weight, and density might be responsible for its no effect observed in this study [11, 14]. The limitation of the use of mirtazapine in this study was the lack of evaluation for its prokinetic effect on large intestine movements to specifically check whether it might have any effect. A round shaped foreign body will not be influenced too much by secondary factors noted above and come out as a stool. If the effect of mirtazapine on the ability of movement in large intestine during capsule endoscopy is confirmed, it will provide much clear result on the influence of GTT by factors other than mechanisms of mirtazapine [11, 22]. The current veterinary clinical trial’s technical standards make it difficult to perform capsule endoscopic examination for the large intestine. In human medicine, clinical trials of capsule endoscopic examinations of large intestine are currently undergoing in the United States of America and Europe. To have future application in veterinary clinical trials, capsule endoscopy of prokinetics such as mirtazapine that can enhance large intestine motility will be needed [23].
Capsule retention is a major side effect that causes imperfect examination of capsule endoscopy. In human medicine, a capsule retention is defined when the capsule remains in the gastrointestinal tract for more than two weeks [6]. Previous researches conducted in veterinary medicine have reported more cases and probabilities of capsule retention in dogs compared to those in humans [7, 8]. In human medicine, Crohn’s disease patients who had confirmed small intestine lesion and child patients with body mass index less than 5percentile, occurrence rates of capsule retention were 37.5% and 43%, respectively [24]. There are reports that such capsule retention has no relevance with dog’s body size. However, research conducted in veterinary medicine lacked parameters mostly. In addition, the smaller the patient’s size, the smaller the gastrointestinal inner diameter. This could play as a factor on capsule retention. Therefore, considerations and attention must be paid to smaller patient with small intestine disease [8, 24].
GITT of capsule endoscopy in dogs is currently unclear. In a previous research conducted in veterinary medicine where it targeted 40 healthy beagle dogs as a research group, complete examination rate of gastrointestinal tract was 57% [7]. On the other hand, a research conducted on 8 dogs with digestive symptoms showed complete examination rate of 37.5% [8]. However, the complete examination rate was 100% in this study. In one of these two studies on dogs with gastrointestinal symptoms, the cause of incomplete examination of capsule endoscopy was capsule retention. All of them had extensive gastroenteropathy [8]. The correlation of gastrointestinal related disease with capsule retention is currently unclear in dogs. However, it has been proven that capsule retention has higher occurrence in patients with Crohn’s disease and tumor in human medicine. Thus, gastrointestinal disorder or delayed gastric emptying might be the cause of capsule retention [6]. However, such research has low number of cases with bias in statistics for patient selection, making it difficult to reach a solid argument on clear correlations [6]. There was no occurrence of capsule retention in this study, although longer retentive time within the stomach meant longer time it took to pass through the gastrointestinal tract. Although the effect of delayed emptying within stomach on capsule retention could not be evaluated in this study, delayed empty was still identified as a major factor that influenced the movement of the capsule (Table 2).
In veterinary medicine, Davignon et al. stated that capsules being moved within the gastrointestinal tract by peristalsis varied from 1 minutes to 270 minutes for capsule to pass the stomach. For small intestines, the movement time of individuals ranged from 15 minutes to 180 minutes [8]. The present study also showed great difference between individuals, with GTT varying from 60 minutes to 330 minutes and SBTT varying from 30 minutes to 210 minutes. On each research excluding the individual that showed capsule retention, the measured GITT was 35 minutes to 420 minutes. In this research, it was confirmed to be 90 minutes to 420 minutes. Therefore, if there is no retention within the stomach, the capsule will safely pass through most of the dog’s total gastrointestinal tract within 12 hours of battery life. However, other correlations that could influence the overall gastrointestinal passing rate such as breed, weight, age, or gender were not considered in this study. Therefore, additional assessment is required to determine possible differences in individual's small intestine length and bowel movement.
This study has some limitations. First, it had a low number of research population. It was conducted on healthy male beagle dogs. There may not be a sign of effect on dogs with normal gastric emptying capability when using metoclopramide. To make its effect evident, additional experiments with reduced gastrointestinal motor function environment are needed. A follow up study with larger research population is also needed. The Olympus Endo Capsule® (Olympus Inc., Tokyo, Japan) used for the previous veterinary study had diameter of 11×26 mm whereas a relatively smaller capsule of 10.8×25.5 mm was used in this study [8]. Despite such difference, there were no significant results in the present study. Thus, capsule that may further reduce the influence caused by secondarily factors such as capsule’s size, composition, weight, and density should be developed.