Introduction
Neurogenesis and neuronal migration are essential development steps in the nervous system. Neurogenesis is maximally active in the embryo. Neurogenesis gradients offer clues to where in the neuroepithelium the germinal site may be located and what routes migrating cells take to reach their final destination [1, 2]. Neurons and nonneuronal cells originate from the site of their last mitotic division such as the ventricular zone. They migrate to their final destination and become integrated into specific brain circuits. Thus, neuronal migration is also a key development process in the central nervous system [2–7].
Dopaminergic neurons are one of the major neuronal populations in the ventral mesencephalon [8]. Dopaminergic neurons are distributed in the three specific lesion in the ventral mesencephalon: substantia nigra (SN), ventral tegmental area (VTA), and retrorubal field (RRF) [9]. They function as excitatory neurotransmitter and related with diverse brain functions including motor control, emotion, cognition, and reward behaviors [10, 11]. They play important roles in the regulation of dopaminergic neuron activity and striatal and cortical neurons [12].
Neurogenesis and neuronal migration of dopaminergic neurons are not well understood yet. The present study demonstrated early and later neurogenesis and neuronal migration of dopaminergic neurons in the mouse mesencephalon.
Materials and Methods
Timed pregnant CD1 mice were purchased from Charles River Laboratories (Wilmington, MA, USA) and were maintained in an animal facility. A female mouse housed with a male mouse for 15–17 hours was examined for the presence of vaginal plugs at 9:00 A.M. The presence of the plug indicated conception. The day of plug discovery was designated embryonic day 0 (E0). Animal experiments were in full compliance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the McLean Institutional Animal Care and Use Committee.
To understand neurogenesis, a single 5-bromodeoxyuridine (BrdU: 50 μg/g body weight, i.p; Sigma) injection was administrated to E10, E11, E12, E13, and E14 pregnant dams and each embryo was removed after 24 hours. For neuronal migration study, BrdU labeling was achieved by administering a single BrdU injection (50 μg/g body weight, i.p; Sigma) to pregnant dams carrying E10 or E11 mice. Embryos were removed at E13, E15, E17, and postnatal day 0 (P0) and decapitated. Embryonic brains were immersed in Zinc fixative (BD Pharmingen, USA) for 24 hours and then processed for paraffin wax histology. BrdU immunohistochemistry was performed for 10 μm thick paraffin embedded sections with a mouse monoclonal anti-BrdU antibody (1:75, 347580, BD Pharmingen, USA). Double labeling immunohistochemistry for BrdU and tyrosine hydroxylase (TH) was performed using rabbit TH antibody (1:1,000, P40101-0, Pel-Freez, USA). Double labeling immunohistochemistry for BrdU and TH was performed using sheep polyclonal anti-TH (1:200, AB1542, Millipore, USA). Secondary antibodies used were Streptavidin Alexa fluor 488 conjugate and Streptavidin Alexa fluor 594 conjugate (Molecular Probes, USA).
Results
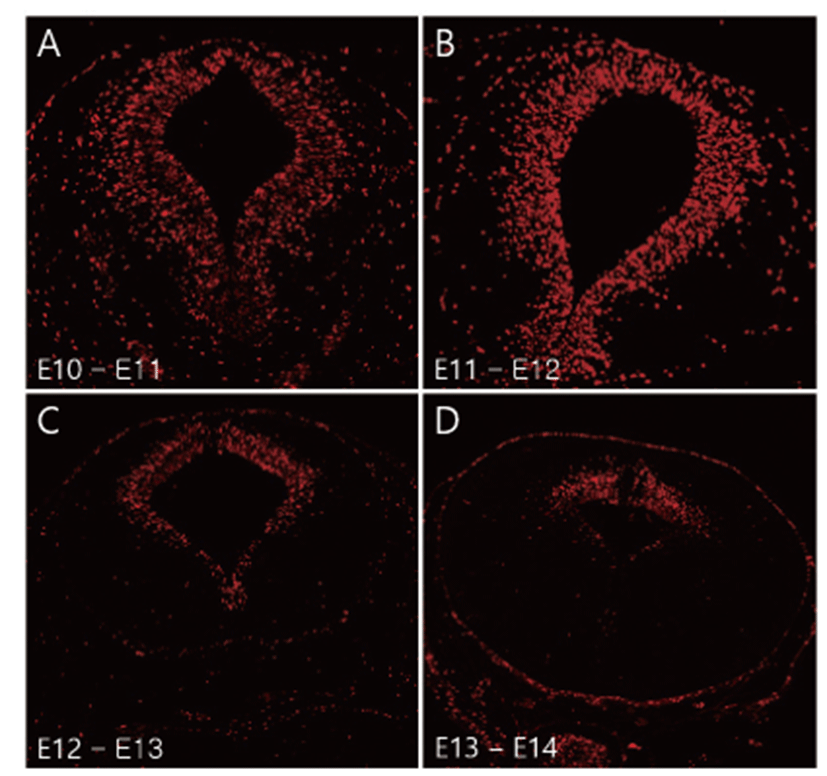
BrdU is integrated into the DNA of S-phase progenitor cells and serves as a stable marker for cells born around the time of injection. Thus, BrdU has been widely used to understand neurogenesis and neuronal migration in the developing brain [13, 14]. To observe mesencephalic neurogenesis, BrdU labelled fetus was collected and immunohistochemistry was performed. Many E10- and E11- originated BrdU positive cells were observed to be uniformly distributed in the whole mesencephalic tegmental neuroepithelium region (Figs. 1A, B). E12-originated BrdU-positive cells were decreased in the ventral region of the mesencephalon compared to E10 and E11- originated cells (Fig. 1C). E13-originated BrdU positive cells were only observed in the dorsal mesencephalon region (Fig. 1D). A few E14-originated BrdU positive cells were detected in the mesencephalon (data not shown).
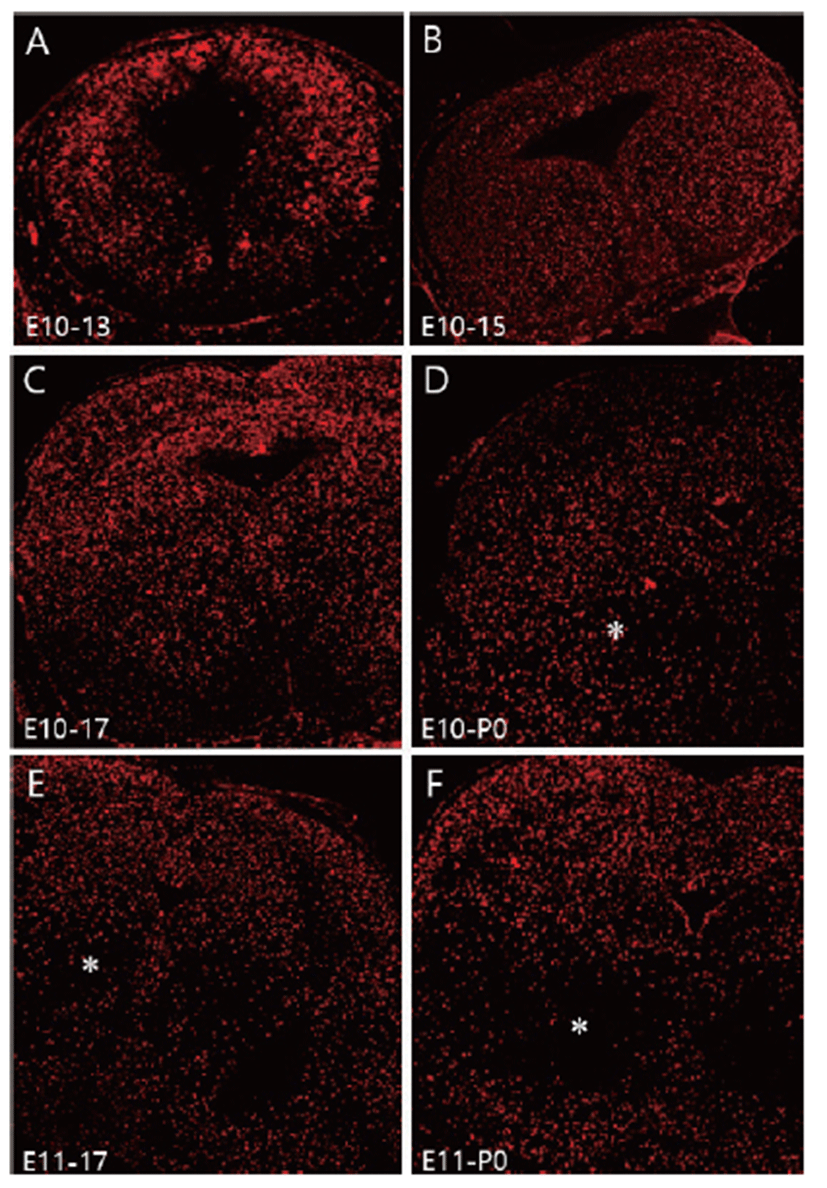
To investigate neuronal migration, a single pulse of BrdU was administrated at E10 and migration of BrdU labeled cells was followed until E13, E15, E17, and P0. E10-labeled BrdU positive cells spread out uniformly in the mesencephalon from E13 to E17 (Figs. 2A–C). Distribution of E10-labeled BrdU positive cells were slightly depleted at P0 (Fig. 2D), at which time the number of neurons located in the red nucleus area had diminished and these neurons had migrated in both ventral and dorsal directions. Changed distribution pattern was clearly seen in E11-labeled BrdU positive cells (Figs. 2E, F). The distribution pattern of E11-labeled BrdU positive cells was similar to that of E10-labeled BrdU cells until E15 (data not shown). However, a depletion of E11-labeled BrdU positive cells was clearly seen at E17 (Fig. 2E), and the majority of E11-labeled BrdU positive cells had depleted at P0 in the red nucleus region (Fig. 2F). This neuronal depletion in the red nucleus area seems to be a consequence of ventral and dorsal neuronal migration of E11- labeled BrdU positive cells. Although a slightly different neuronal migration was observed between E10- and E11- labeled cells, they showed the same dorsal and ventral neuronal migration pattern in the mesencephalon.
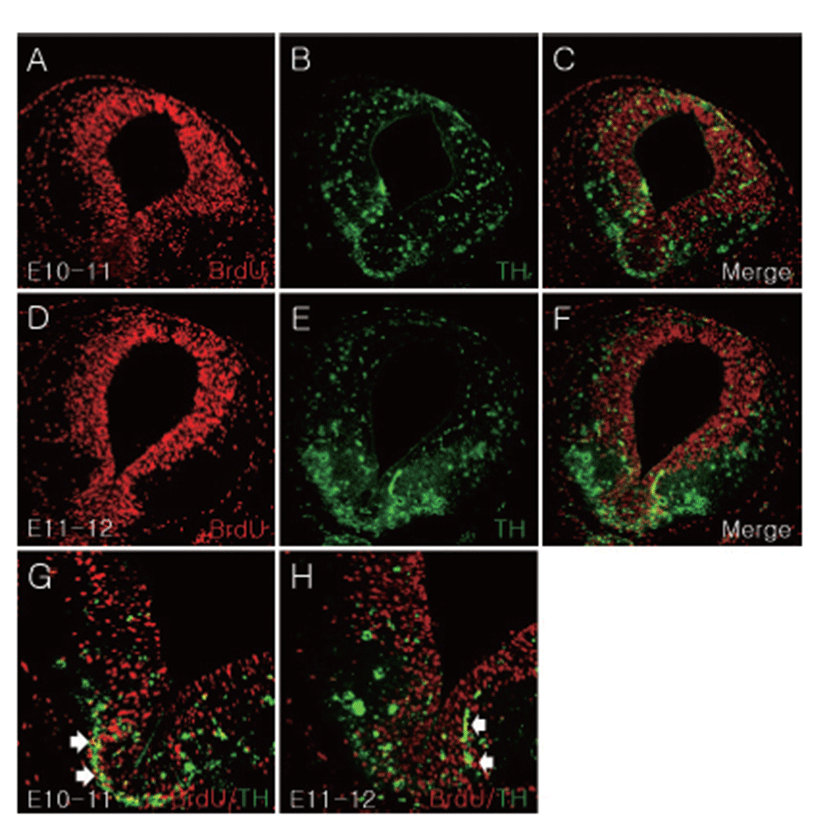
Dopaminergic neurons in the ventral midbrain organized three specific lesions: RRF, SN, and VTA [2, 15]. To investigate when and where dopaminergic neurons were formed in the ventral mesencephalon, BrdU was injected into CD1 mice at E10, E11, E12, E13, and E14. Each brain sample was obtained after 24 hours and migration of E10-labeled cells observed at E17. TH/BrdU double positive cells were identified in the mesencephalic region (Fig. 3). E10-labeled TH positive cells were spread out uniformly in the mesencephalon, and TH and BrdU double labeled cells were identified in ventromedial mesencephalic region (Figs. 3B, C, G). E11-labeled TH positive cells were confirmed in ventral mesencephalon (VM), and TH and BrdU double labeled cells were identified in VM (Figs. 3E, F, H).
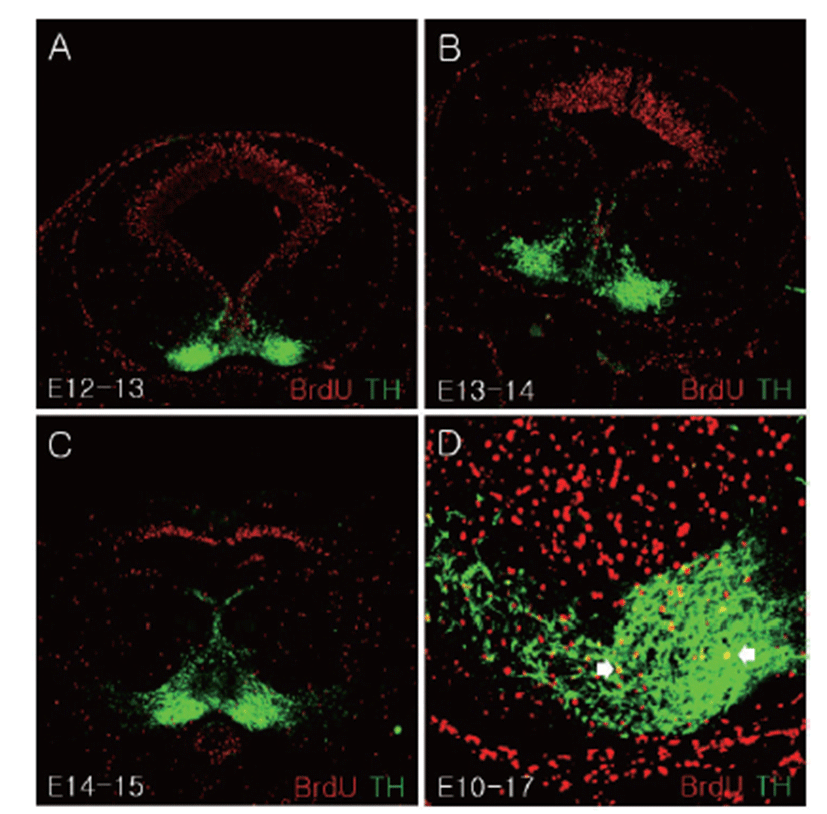
In the cells labeled with BrdU after E12, most BrdU positive cells were observed in the dorsal mesencephalon region whereas TH positive cells were mainly confirmed in the ventral mesencephalon (Figs. 4A–C). E10-labeled BrdU positive cells were merged with TH positive cell at E17 in the SN region (Fig. 4D).
Discussion
Neurogenesis study using [3H] thymidine has shown that neurogenesis mainly occurs at E10 and E12 in the mouse mesencephalon [16]. The results of the neurogenesis study using BrdU corroborated the previous study indicating that neurogenesis is generated predominantly at E10 and E11. BrdU positive cells were observed to be uniformly distributed in the whole mesencephalon at E10-11 and E11-12 in this study. This distribution pattern was no longer observed after E13-labeled BrdU, and BrdU positive cells were only detected in the restricted dorsal mesencephalon after E13-labeled BrdU.
In a previous TH study, TH mRNA was detected at E11.5 by in situ hybridization and real time reverse transcription-PCR [17]. A dopamine and cyclic adenosine- 3':5'-monophosphate-regulated phosphoprotein with an apparent molecular weight of 32 kilodaltons (DARPP-32), a pre-requisite for the dopaminergic neurons development, was detected at the E-12 [18]. Our results coincided with the previous studies. TH expression was initially detected at E11 and many E10-labeled BrdU positive cells were found to be merged with dopaminergic neurons in the SN and VTA at E17. These results demonstrated that dopaminergic neuronal development begins at E10.
Neuronal migration seems to be an essential phase in the formation of the anatomical architecture of the mesencephalon. One important feature of neuronal cells in the developing central nervous system is their ability to migrate away from their origin germinal zone to their final position [4]. Cells generated in the ventral mesencephalon can migrate ventrally and dorsally [19]. This BrdU study showed evidence of neuronal migration in the mesencephalon. E10- and E11-labeled BrdU positive cells were uniformly distributed at E11-E12. However, these cells become diminished in the red nucleus area at E17. This depletion of cells is considered to be the consequence of dorsal and ventral neuronal migration. Although a minor difference was observed between the migration of E10- and E11-labeled cells, they showed the same dorsal and ventral migration pattern. It has been shown that E11-originated neurons can migrate ventrally to form SN and VTA [19, 20]. This study showed similar result in that E10- and E11-BrdU labeled cells merged with TH labeled cells were detected in the SN and VTA at E17.