Original Article
Clinical characteristics and outcomes in patients with lesion-positive transient ischemic attack
Su-Jeong Kang, Sang-Gil Lee, Kyu Sun Yum, Ji-Seon Kim, Sung-Hyun Lee, Sang-Soo Lee, Dong-Ick Shin*

Department of Neurology, Chungbuk National University College of Medicine and Medical Research Institute, Chungbuk National University Hospital, Cheongju 28644, Korea
*Corresponding author: Dong-Ick Shin Department of Neurology, Chungbuk National University College of Medicine and Medical Research Institute, Chungbuk National University Hospital, Cheongju 28644, Korea Tel: +82-43-269-6372, Fax: +82-43-273-7591, E-mail:
sdi007@hanmail.net
© Research Institute of Veterinary Medicine, Chungbuk National University. All rights reserved.
Received: Sep 27, 2018; Accepted: Dec 24, 2018
Abstract
Transient ischemic attack (TIA) indicates high risk for major stroke and is considered a medical emergency. Diffusion-weighted imaging (DWI) enables detection of acute ischemic lesions. The clinical significance of DWI positive lesions in TIA is obscure and its prevalence, clinical features are not established. Therefore, we performed a clinical, etiological and prognostic analysis through a cross-sectional analysis of 235 TIA patients, grouped according to presence of DWI lesion. Clinical features, underlying risk factors for stroke, outcome and rate of recurrence were analyzed. 3 months follow-up of modified Rankin Scales (mRS) were done with telephone survey. DWI positive lesions were present in 14.0% of patients. Etiological factors significantly associated with DWI lesions in TIA patients were male sex (p = 0.038), stroke history (p = 0.012) and atrial fibrillation (p < 0.001). Presence of at least one medium or high risk of cardioembolism from TOAST classification were not associated with lesions when excluding association to atrial fibrillation (p = 0.108). Clinical features showed no significant difference. Whether the patients had lesion-positive DWI was not related to an increase in mRS score during the hospital stay or at the 3-month follow-up after discharge. Future studies should include multi-center samples with large numbers, considering each unique medical environment. Routine acquisition of follow-up DWI for proper evaluation of the tissue-based definition of TIA should also be considered.
Keywords: transient ischemic attack; diffusion weighted imaging; cardioembolism; stroke; atrial fibrillation
Introduction
Individuals who experience a transient ischemic attack (TIA) are at high risk of stroke [1–3]. The stroke incidence is ~10% at 90 d after TIA and ~15% at 1 year after TIA [1]. The 1988 World Health Organization (WHO) criteria define TIA as focal neurological symptoms lasting less than 24 h with no clear non-vascular cause [4]. Diffusion- weighted imaging (DWI) enables detection of acute ischemic lesions [5, 6]. The clinical significance of the presence of such lesions in patients with TIA has gained recent attention, as acute ischemic lesions are reportedly independently predictive of stroke [7–10]. Therefore, a tissue-based definition of TIA (i.e., focal neurological symptoms lacking acute ischemic lesions) is emerging [11–14].Clinical features of patients with lesion-positive DWI are unclear, and findings vary [15–21]. Clinical and etiological characteristics of patients with TIA and lesion-positive DWI have been examined. A systematic review of 19 studies (combined n = 1,242; individual studies, median n = 58, range n = 14–200) concluded that atrial fibrillation and ipsilateral carotid stenosis of >50% were associated with lesion-positive DWI, whereas age, sex, hypertension, and diabetes history were not [22]. Symptoms of motor weakness, dysphagia, and dysarthria were associated with lesion-positive DWI, as was symptom duration >60 min [22]. Conversely, the rate of lesion-positive DWI was not significantly related to the presence of atrial fibrillation in a Japanese study of 464 patients [23]. Therefore, the significance of the lesionpositive DWI is unclear.
Here, we assessed the clinical characteristics, features, and outcomes of lesion-positive DWI in patients with TIA admitted to a stroke center.
Materials and Methods
AThis was a retrospective study on a prospectively collected stroke database. We evaluated 235 patients with TIA who visited our stroke center between January 2009 and December 2015. Vascular neurologists made diagnoses in accordance with the WHO criteria, which define TIA as an acute loss of focal cerebral function lasting less than 24 h [4]. DWI was performed routinely in all patients with TIA upon admission (i.e., within 24 h of symptom onset) if the patient had no contraindications for magnetic resonance imaging (MRI). Lesion-positive DWI was defined as an increased signal on DWI with a reduced apparent diffusion coefficient.
Department of Neurology physicians recorded detailed baseline data for patients with TIA including demographics, clinical features, imaging features, and precise potential sources of cardioembolism, per the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification [24]. Modified Rankin scale (mRS) scores were acquired at admission and at discharge from the hospital [25]. All data collected were recorded prospectively in the medical center registry; the 3-month follow-up mRS score was determined via a telephone survey administered by a research nurse affiliated with the Department of Neurology [26]. For categorical variables, Pearson’s chi-square test and Fisher’s exact test were used to determine correlations with lesion-positive DWI. Association of lesion-positive DWI with cardioembolic risk stratification according to TOAST classification was calculated by excluding the effect of atrial fibrillation using the Cochran–Mantel–Haenszel test. Relative risk associated with each variable for lesion-positive DWI was calculated. For continuous variables, Student’s t-test was used. Statistical analysis was performed using SPSS software.
Results
The proportion of patients with lesion-positive DWI was 14.0% among 235 subjects with TIA. Continuous etiological variables are presented in Table 1, and categorized variables are presented in Table 2. Only male sex, history of previous stroke, atrial fibrillation, and medium- to high-risk source of cardioembolism (TOAST classification) were found to be significantly associated with the presence of lesion-positive DWI. Atrial fibrillation showed the highest relative risk of cardioembolism. Medium- to high-risk sources of cardioembolism were not significantly related to lesion-positive DWI when the presence of atrial fibrillation was excluded (p = 0.108; Cochran–Mantel–Haenszel test). Although not significant, a trend toward association of sources of high-risk for cardioembolism (TOAST classification) with lesionpositive DWI was noted if atrial fibrillation was excluded (p = 0.052; Cochran–Mantel–Haenszel test). No association between the presence of lesion-positive DWI and clinical features was found (Table 3). Whether the patients had lesion-positive DWI was not related to an increase in mRS score during the hospital stay or at the 3-month follow-up after discharge (Table 4).
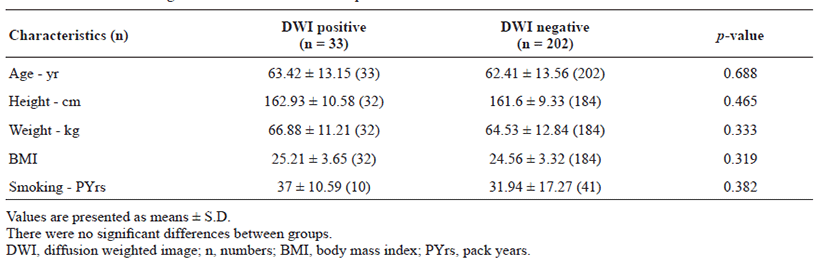
Table 1.
Continuous etiological variables in association to positive DWI
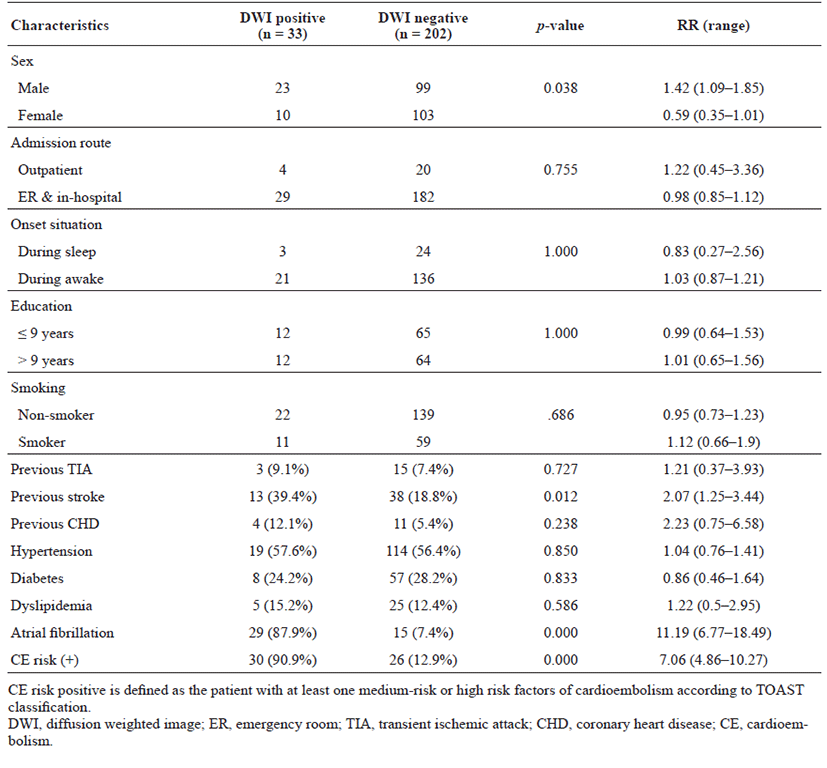
Table 2.
Categorized etiological variables in association to positive DWI
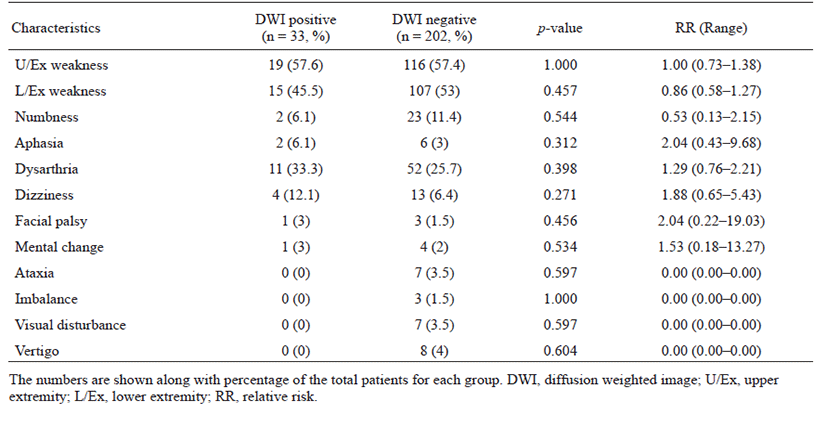
Table 3.
Clinical features in association to positive DWI
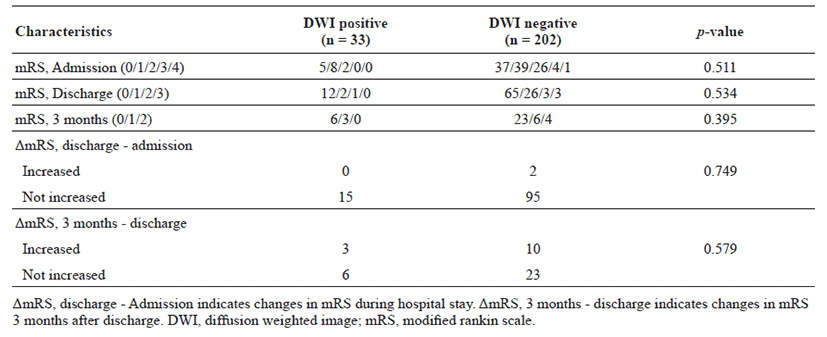
Table 4.
Prognosis in association to positive DWI
Discussion
We found that etiological factors associated with a high risk of stroke were also associated with the presence of lesion-positive DWI in patients with TIA. These factors included male sex, previous history of stroke, and presence of atrial fibrillation. A high risk of cardioembolism according to TOAST classification showed a trend toward association with lesion-positive DWI, but the relationship was not statistically significant when atrial fibrillation was excluded. No clinical features were associated with the presence of lesion-positive DWI. These findings differ from the previously reported large systematic review, which showed that traditional vascular risk factors (age, sex, diabetes, hypertension, and blood pressure) were not associated with lesion-positive DWI, while clinical features, such as motor weakness, dysarthria, aphasia, and time from onset > 60 min, were [22]. Although the association with the time from onset was not assessed in this study, the admission route and symptom onset during sleep or waking were assessed (Table 2). Patients admitted via the outpatient clinic and those with symptom onset noted during sleep likely underwent DWI longer after onset than did inpatients and those who first experienced symptoms while awake. However, no significant association was detected.
The differential findings may be due to differences in ethnicity, as well as differences in the medical environment. The hospital environment affected the time from onset to radiological assessment in an observational study [27]. Detection of ischemic lesions is difficult 1–12 h after TIA; therefore, different treatment and imaging protocols in different medical environments may affect detection of lesion-positive DWI in patients with TIA [28]. On the other hand, in a study of multi-modal MRI in patients with TIA, new lesions were found in 46.7% of patients who initially showed normal DWI findings at a follow-up a few days after onset [29]. Therefore, DWI findings in the acute TIA setting may not represent the presence of true tissue ischemia; variations in the time from onset and lack of DWI follow-up may be a limitation of current reports.
For prognosis, the mRS score was analyzed according to the presence of lesions on DWI, and no significant effect was noted. This is in accordance with a systematic review on outcomes of patients with non-disabling stroke, where the prognosis was similar between the DWI-negative and -positive groups [30], but contrary to previous reports where lesion-positive DWI was independently predictive of stroke recurrence [7–9].Several limitations of this study should be noted. First, this study was a retrospective analysis of patients with TIA. Second, this study had a small sample size, which limited the statistical power, and it was performed at a single center.
In conclusion, the etiological factors associated with lesion-positive DWI in patients with TIA were male sex, previous stroke history, and atrial fibrillation. Clinical features and prognosis were similar between lesion-positive and negative groups. Future studies should include multi-center samples with large numbers, considering each unique medical environment. Routine acquisition of follow-up DWI for proper evaluation of the tissue-based definition of TIA should also be considered.
Acknowledgements
The authors thank all the staff of the Department of Neurology, Chungbuk National University College of Medicine and Medical Research Institute, Chungbuk National University Hospital for their helpful contribution.
References
Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a populationbased study. Neurology 2004;62:2015-2020.


Nguyen-Huynh MN, Johnston SC. Evaluation and management of transient ischemic attack: an important component of stroke prevention. Nat Rev Cardiol 2007;4:310-318.


Giles MF, Albers GW, Amarenco P, Arsava EM, Asimos AW, Ay H, Calvet D, Coutts SB, Cucchiara BL, Demchuk AM, Johnston SC, Kelly PJ, Kim AS, Labreuche J, Lavallee PC, Mas JL, Merwick A, Olivot JM, Purroy F, Rosamond WD, Sciolla R, Rothwell PM. Early stroke risk and ABCD2 score performance in tissuevs time-defined TIA: a multicenter study. Neurology 2011;77:1222-1228.



The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol 1988;41:105-114.

Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EFM. Guidelines for the early management of adults with ischemic stroke. Circulation 2007;115:e478-e534.


Yilmaz U. Diffusion-weighted imaging in acute stroke. Radiologe 2015;55:771-774.


Calvet D, Touze E, Oppenheim C, Turc G, Meder JF, Mas JL. DWI lesions and TIA etiology improve the prediction of stroke after TIA. Stroke 2009;40:187-192.


Coutts SB, Simon JE, Eliasziw M, Sohn CH, Hill MD, Barber PA, Palumbo V, Kennedy J, Roy J, Gagnon A, Scott JN, Buchan AM, Demchuk AM. Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann Neurol 2005;57:848-854.


Purroy F, Montaner J, Rovira A, Delgado P, Quintana M, Alvarez-Sabin J. Higher risk of further vascular events among transient ischemic attack patients with diffusion-weighted imaging acute ischemic lesions. Stroke 2004;35:2313-2319.


Gon Y, Sakaguchi M, Okazaki S, Mochizuki H, Kitagawa K. Prevalence of positive diffusion-weighted imaging findings and ischemic stroke recurrence in transient ischemic attack. J Stroke Cerebrovasc Dis 2015;24:1000-1007.


Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease: the American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40:2276-2293.


Mohr JP. History of transient ischemic attack definition. Front Neurol Neurosci 2014;33:1-10.

Siket MS, Edlow J. Transient ischemic attack: an evidence- based update. Emerg Med Pract 2013;15:1-26.

Uno H, Taguchi A, Oe H, Nagano K, Yamada N, Moriwaki H, Naritomi H. Relationship between detectability of ischemic lesions by diffusion-weighted imaging and embolic sources in transient ischemic attacks. Eur Neurol 2008;59:38-43.


Wardlaw J, Brazzelli M, Miranda H, Chappell F, Mc- Namee P, Scotland G, Quayyum Z, Martin D, Shuler K, Sandercock P, Dennis M. An assessment of the cost-effectiveness of magnetic resonance, including diffusion-weighted imaging, in patients with transient ischaemic attack and minor stroke. a systematic review, meta-analysis and economic evaluation. Health Technol Assess 2014;18:1-368.



Anticoli S, Pezzella FR, Pozzessere C, Gallelli L, Bravi MC, Caso V, Siniscalchi A. Transient ischemic attack fast-track and long-term stroke risk: Role of diffusionweighted magnetic resonance imaging. J Stroke Cerebrovasc Dis 2015;24:2110-2116.


Vilanova MB, Mauri-Capdevila G, Sanahuja J, Quilez A, Pinol-Ripoll G, Begue R, Gil MI, Codina-Barios MC, Benabdelhak I, Purroy F. Prediction of myocardial infarction in patients with transient ischaemic attack. Acta Neurol Scand 2015;131:111-119.


Scheef B, Al-Khaled M. Atrial fibrillation in patients with transient ischemic attack in accordance with the tissue-based definition. J Vasc Interv Neurol 2016;9:23-27.


Chang JY, Kim dH, Chung JH, Yum KS, Hong JH, Han MK. Hospital-based prospective registration of acute transient ischemic attack and moncerebrovascular events in Korea. J Stroke Cerebrovasc Dis 2015;24:1803-1810.


Redgrave JNE, Coutts SB, Schulz UG, Briley D, Rothwell PM. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke 2007;38:1482-1488.


Hama Y, Uehara T, Ohara T, Kimura K, Okada Y, Hasegawa Y, Tanahashi N, Suzuki A, Takagi S, Nakagawara J, Arii K, Nagahiro S, Ogasawara K, Nagao T, Uchiyama S, Matsumoto M, Iihara K, Toyoda K, Minematsu K. Clinical characteristics of transient ischemic attack patients with atrial fibrillation: analyses of a multicenter retrospective study. Cerebrovasc Dis Extra 2015;5:84-90.



Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35-41.


Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified rankin scale. A systematic review 2009;40:3393-3395.

Uno H, Nagatsuka K, Kokubo Y, Higashi M, Yamada N, Umesaki A, Toyoda K, Naritomi H. Detectability of ischemic lesions on diffusion-weighted imaging is biphasic after transient ischemic attack. J Stroke Cerebrovasc Dis 2015;24:1059-1064.


Nah HW, Kwon SU, Kang DW, Lee DH, Kim JS. Diagnostic and prognostic value of multimodal MRI in transient ischemic attack. Int J Stroke 2014;9:895-901.





