Introduction
A saccular aneurysm (SA) is a localized weakness of the arterial wall with a characteristic berry-like and outpouched shape. It is the most common form of intracranial aneurysm. In humans, intracranial SA occurs in 1-2% of the population, and in 80-85% of cases it leads to non-traumatic subarachnoid hemorrhage [1]. Mortality rates of subarachnoid hemorrhage have been reported to be 9.8% from day 0 to 14 and 19% at 3 months [2]. Therefore, early detection of intracranial SA and prevention of rupture are important. Interventional options in treating intracranial SA include surgical clipping and endovascular management, such as aneurysmal coiling [1].
Various methods have been developed to create SA models in experimental animals [3]. There are surgical models where an arteriovenous shunt is created or where the jugular vein is connected to the common carotid artery with a mechanically attached venous graft. Large aneurysms can be caused using surgical methods, however, the venous characteristics of such induced aneurisms are different from those of real aneurysms [4, 5]. Furthermore, a traumatic model created through a puncture or excision and a chemical injury model created by using toxic or necrotizing agents have characteristics that are different from those of a human aneurysm, and both models can lead to thrombosis, rupture, or calcification at the aneurysmal site [3].
An enzymatic degradative aneurysm model can be created by using porcine pancreatic elastase or papain. An animal model of porcine pancreatic elastase-induced aneurysm was first developed decades ago, and elastase-induced models are widely used even until now. Elastase leads to apoptosis of the arterial wall via the intrinsic pathway; the arterial wall then develops an aneurysm [6]. Elastase-induced rabbit SA models have hemodynamics and geometric features that are similar to those of human cerebral aneurysms [7, 8]. A recent study investigating un-ruptured aneurysms revealed that the average size of an un-ruptured aneurysm is about 6 mm in humans. This size has not been achieved in elastase-only induced models [9]. Also, the elastase-only SA model is not sufficient to create aneurysms because it thickens the arterial wall, which makes the resulting aneurysm different from a human late stage aneurysm [10]. Therefore, many attempts to create large aneurysms combined with transplantation have been made.
Papain, a proteolytic enzyme from papaya latex, breaks down peptide bonds [11]. Because of its strong proteolytic properties, it is widely used in experimental animals to create chronic obstructive pulmonary models, pulmonary emphysema models, osteoarthritis models, and disk degeneration models [12-16]. Papain was also recently used to create SA models. A recent study to create a rabbit SA model using papain found that papain yielded an aneurysm of approximately 7 mm in diameter. This aneurysm has histologic similarities with elastase-induced aneurysms that have features similar to those of human aneurysms [16]. A comparison between histopathologic evaluations of elastase-induced and papain-induced aneurysms is also needed; however, such a comparison has never been conducted. Our study therefore aims at comparing the efficiency of elastase-induced and papain- induced SA models in rabbits as well as the differences between both models.
Materials and Methods
This study was approved by the ethical committee of the Gyeongsang National University Laboratory Animal Center (approval number: GNU-140729-D0030). Animals were treated according to the “Guide for the care and use of laboratory animals” [17].
Eleven New Zealand white rabbits (six male and five female) weighing 1.5 to 2.0 kg (9-10 weeks old) were provided by SAMTAKO (Osan, Gyeonggi-do, South Korea). The rabbits were randomly divided into three treatment groups: group 1, control group (n=3, 0.9% normal saline, 0.1 mL); group 2, 1 mg papain-induced group (n=4, papain from papaya latex, buffered aqueous suspension, 16-40 units/mg protein, P3125, Sigma-Aldrich, St. Louis, USA); group 3, 1 mg elastase-induced group (n=4, elastase from porcine pancreas, Type I ≥ 4.0 units/ mg protein, P1250, Sigma-Aldrich, St. Louis, USA). Papain was diluted with normal saline to reach the same volume (0.15 mL) as elastase.
The rabbits had been fasted for about 3 hours before the operation. They were premedicated with medetomidine (0.2 mg/kg, administered subcutaneously [s.c.], Domitor®; Pfizer, NY, USA) together with acepromazine (0.1 mg/kg, s.c., Sedaject®; Samu Median, South Korea). Glycopyrrolate (0.01 mg/kg, SC, Mobinul®; Myungmoon, South Korea) was administered only when the heart rate dropped below the lower margin of the normal range. After premedicant administration, pre-oxygenation was prepared with 100% O2 before the induction of anesthesia. Enrofloxacin (5 mg/kg, s.c., Baytril®; Bayer, Germany) was used as a prophylactic antibiotic and meloxicam (0.2 mg/kg, s.c., Metacam®; Boehringer Ingelheim, Germany) was used as an anti-inflammatory analgesic agent. Normal saline was administered at 10 mL/kg/hour through the marginal ear vein during the whole procedure. Heparin (100 U/kg, administered intravenously (i.v.); Heparin Sodium Injection®; Hanlim, South Korea) was administered before the operation to prevent clot formation. After 15 minutes had passed from the administration of the premedicants, a face mask with a diaphragm was used, and isoflurane (Ifran®; Hana pharm, South Korea) was used both as an induction agent and also as a maintenance agent. The rabbit’s heart rate, body temperature, and percutaneous blood oxygen saturation (SpO2) was monitored during anesthesia. A circulating water blanket (Medi-Therm®, Gaymar, NY, USA) with a temperature of 38-39°C was used to maintain body temperature. Ophthalmologic ointment was applied to protect the eye from drying out during anesthesia.
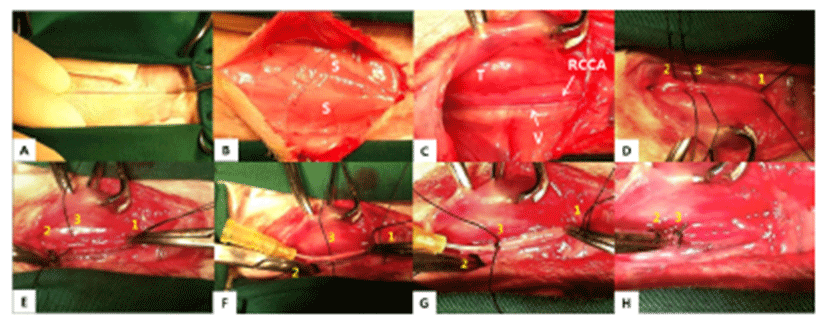
Surgery was conducted as previously described (de Oliveira et al.) [16]. The whole surgical procedure was performed using a sterile technique and surgical instruments were sterilized using the autoclave or ethylene oxide. Rabbits were positioned ventro-dorsally and the neck was fully extended. The hair was clipped from the caudal part of the mandible to the middle of chest. The surgical site was disinfected with 0.1% povidone-iodine and isopropyl- alcohol. A mid-line skin incision was performed from the middle of the neck to the manubrium. The skin, fascia, and muscles were divided at the right side of the trachea and the carotid sheath was exposed. The right jugular vein and the vagus nerve were separated from the right common carotid artery (RCCA). Right in front of the manubrium, the blood flow was temporarily blocked with a Double (Potts) loop using a mosquito hemostat [first thread, caudal]. Two silk threads were placed cranially. Of one of the two threads, the most cranial thread [second thread, cranial], was sutured with a simple interrupted pattern to prevent the back flow of blood. A 24 G was placed in between the two cranial threads [second and third thread] and the third thread was tied to the vessel with the catheter to prevent leakage. The first and third threads were knotted with a 13 mm interval in between. Trapped arterial blood was suctioned and washed with 0.9% normal saline. The substances (papain, elastase, or normal saline) were applied and were incubated for 20 minutes. Papain and elastase were kept under refrigeration and incubated at a temperature of 36°C shortly before use. Moist gauzes were provided during incubation, to protect the operation site from drying due to the surgical light. After 20 minutes, the applied substances were suctioned and the catheter was removed. The middle thread was sutured firmly with a transfixation ligature and the caudal thread was removed. Influx of arterial blood to the aneurysmal site was checked and confirmed. When no hemorrhage or leakage was detected, the surgical site was closed. The muscle layer (simple interrupted suture) and subcutaneous layer (continuous suture) were sutured with PDS II 4-0 (Ethicon®, NJ, USA) and the skin was closed using a skin stapler (Covidien®, MA, USA) (Fig. 1).
The rabbits were observed daily to monitor signs of post-operative complications. Enrofloxacin (5 mg/kg, s.c., once daily [sid]) and meloxicam (0.2 mg/kg, SC, twice daily [bid]) were given for 3 days postoperatively. After medical treatment, the sutured site was disinfected with 0.1% povidone-iodine and was dressed slightly tight with an elastic band [sid] for 3 days. In addition, a soft, cloth-type neck collar was provided to prevent scratching or licking of the surgical site. The stitches were taken out 2 weeks after surgery.
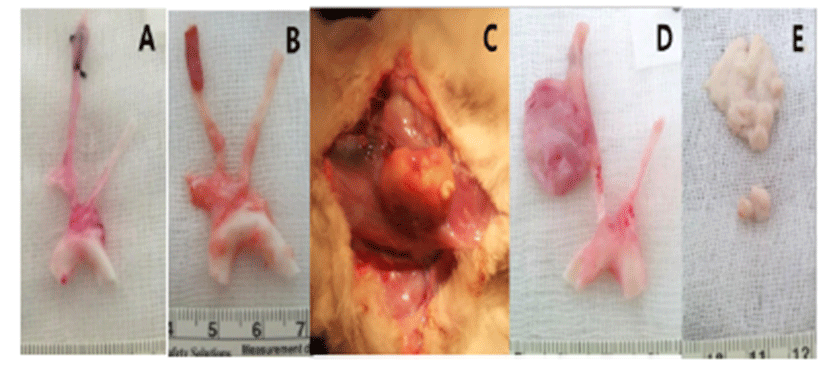
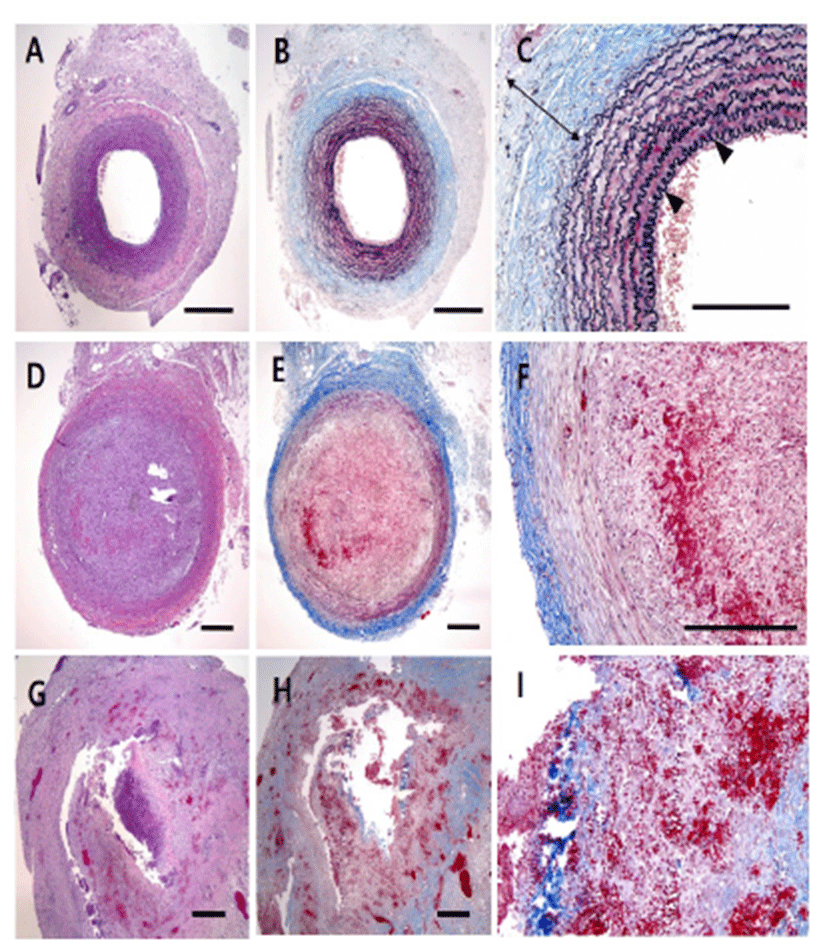
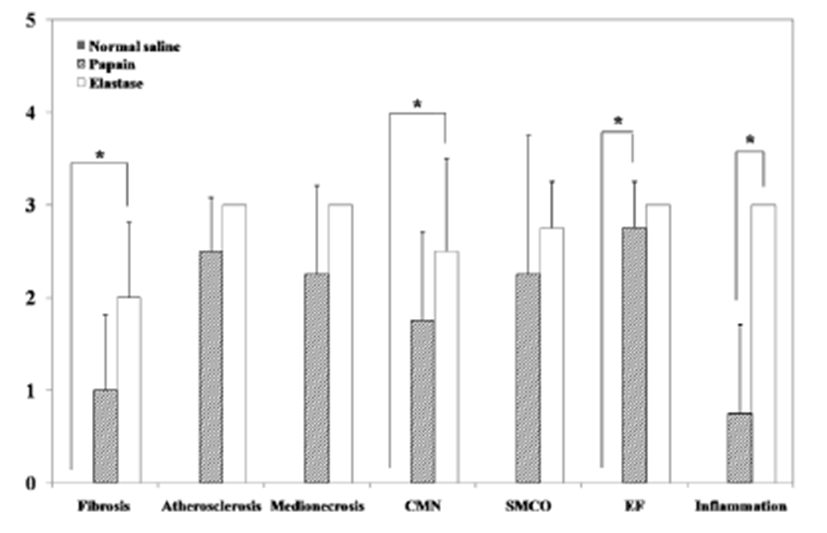
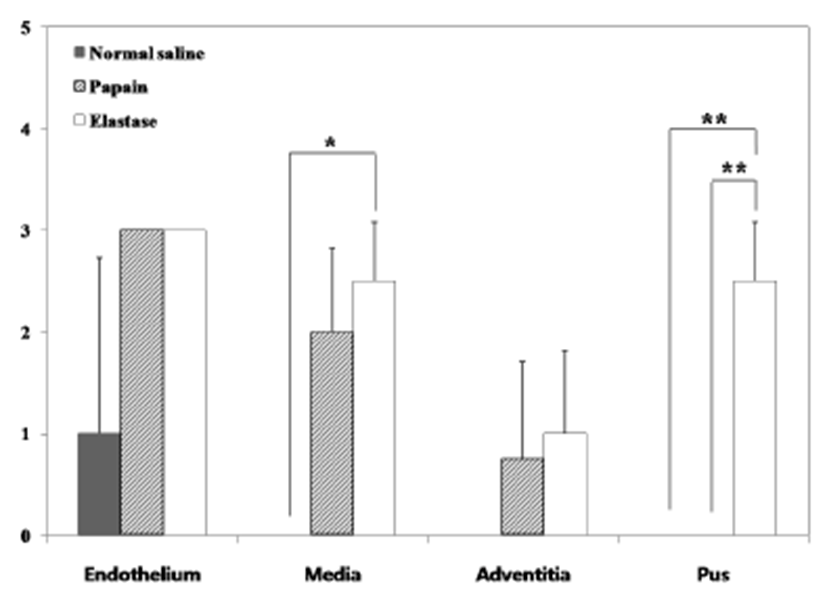
Two weeks after surgery, immediately after the CT examination, etomidate (Lipuro®; B. Braun, Germany), 2 mg/kg i.v., was administered to induce deep anesthesia, followed by potassium chloride, i.v.. Again, a mid-line incision was performed from the cranial part of the neck to the middle of the thoracic cavity. The starting point of the aorta was ligated and dissected. The aortic arch, the brachiocephalic artery, the left common carotid artery (LCCA), and the aneurysmal part of the RCCA were obtained. Harvested vessels were washed with normal saline then fixed with 10% formaldehyde solution. Before staining, samples were sectioned vertically across the long axis. A 2 mm interval transverse sectioning was made, and the middle portions of the samples were selected to be stained. Hematoxylin-eosin (H&E) and modified elastic trichrome stain (Masson’s trichrome and Verhoeff’s stain) were used to observe histologic changes of the muscle tissue, collagen fibers, and elastic fibers. Modified elastic trichrome stain colored muscle tissue red, collagen fibers green, and elastic fibers black [18]. The following histological alterations were analyzed semi-quantitatively: (1) fibrosis (defined as an increase in interstitial collagen); (2) atherosclerosis (defined as the presence of intimal fibrous plaques and/or complex or complicated atheroma); (3) medionecrosis (defined as a focal loss of smooth muscle cell nuclei in the media); (4) cystic medial necrosis (defined as mucoid material accumulation); (5) changes in smooth muscle cell orientation; (6) elastic fragmentation (defined as focal fragmentation of elastic lamellae in the media); and (7) periaortic inflammation (defined as the presence of inflammatory cells). Each variable was graded from 0 (no change) to 3 (most severe change) when examined at a magnification of × 40 or × 200. The criteria for the histological grading were used as proposed by Matthias Bechtel et al. [19], Schlatman and Becker [20], Klima et al. [21] and de Sa et al.[22] and are presented in detail in Table 1. The sum of the results of all variables was calculated for each individual animal, and this was referred to as the vascular wall score.
The diameter of the aneurysm was measured at the largest point. Statistical analysis was performed using SPSS 21.0 (SPSS Inc, Chicago, Ill, USA). Kruskal-Wallis analyses were performed in combination with Dunnett’s posthoc tests by rank transformation of the data, for identifying the relevance of CT width, CT height, CT length, gross width, and gross height in the control, papain, and elastase groups. Values of P < 0.05 were considered to be statistically significant. The results are expressed as means ± standard deviation (SD).
RESULTS
None of the animals died, presented postoperative neurological deficiency, or showed signs of tracheal hemorrhage 14 days after surgery. All rabbits suffered minimal to profound neck and facial swelling, which was reduced within 3 to 5 days after surgery and completely disappeared within 2 weeks. No neurological signs appearednor did any complications occur.
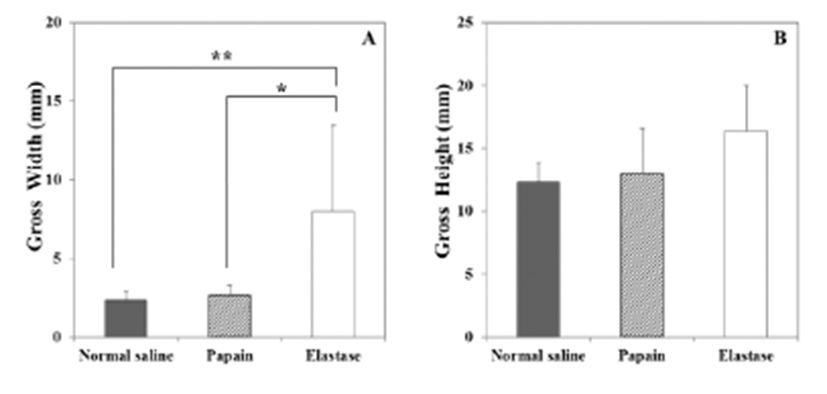
The macroscopic analysis of the control group showed that width and volume of the samples were not different from the LCCA. The papain group showed mild changes including expansion and redness. In all samples that were treated with elastase, various amounts of sterile abscesses in the aneurysmal site were found. The mean gross widths of the aneurysms were 2.3 ± 0.6 mm (control group), 2.9 ± 0.6 mm (papain group), and 8.0 ± 5.5 mm (elastase group). The elastase group was distinctive in that it showed the largest width among all three groups. The mean gross heights of the aneurysms were 12.3 ± 1.5 mm (control group), 13.0 ± 3.6 mm (papain group), and 16.4 ± 3.6 mm (elastase group) (Fig. 2 and 3). The control samples did not present histological alterations, and the internal elastic lamina was visible, showing its characteristic form. To rule out bacterial infections, gram staining was performed. All samples showed negative gram staining, revealing no signs of infections. Notable histological differences were found between the three groups. In accordance with histopathological evaluations in previous studies [19-22], the control group samples showed no histological changes and all layers in these samples remained intact, except for a detachment of the endothelium that occurred in one case (Fig. 4A-C). The papain-treated group showed vascular wall damages including medionecrosis and cystic medial necrosis. All samples had developed elastic fragmentation, and atherosclerosis was present. Fibrosis occurred in three of the four samples and inflammation occurred in half of the samples (Fig. 4D-F). The elastase group met all items of the histopathologic grading criteria and showed severe histologic changes; in particular, sterile abscesses were found (Fig. 4G-I). The results of the histopathological evaluation for animals with papain-induced or elastase- induced aneurysms are listed in Fig. 5 and 6.
Discussion
Several experimental aneurysm models have been developed using mice, rats, rabbits, dogs, pigs, and monkeys [4, 5, 23-28]. Rabbits are the most commonly used experimental animal for SA. Despite their sensitivity to anesthesia and the procedure itself, the size of the common carotid arteries in rabbits is similar to the size of the proximal middle cerebral arteries in humans. Also, the coagulation profile of rabbits is the most similar (among non-primates) to that of humans [5]. Aneurysm models induced by papain have been recently studied in rabbits [16, 28]. Papain was found to be an effective aneurysm- forming agent, but a comparison between papain and elastase under the same conditions has not been carried out. We therefore conducted such a study using rabbits, to compare the results to those of existing studies.
The surgical procedure in this study was suggested by Hoh et al [29]. The RCCA temporarily blocked at the front of the thoracic cavity. However, the caudal part of the external jugular vein that transversely located at that point is particularly vulnerable; thus, great care must be taken during separation. An earlier study[16] reported that the height of the aneurysm could be variable due to the length between the interval of the knots. We therefore conducted ligation at the same interval, about 13 mm, during surgery.
Elastase-induced models are used widely and most studies report that they are successful in creating aneurysms [30, 31]. However, in our study, elastase was found to induce excessive enzymatic destruction leading to the formation of a sterile abscess in the aneurysmal sac. Common reasons for inflammation include microbial agents, toxins, foreign bodies, and others[32][32]. We performed gram staining to confirm whether an infection was present or not, but no signs of infections were found. Many studies [4, 29, 30, 33] that successfully create SA models induced by elastase use rabbits with a weight of 3.0 to 5.0 kg; we used, however, smaller rabbits weighing 1.5 to 2.0 kg. This difference in body weight may cause the concentration of elastase to be higher than in earlier studies, and could be the cause for the induced sterile abscesses we found. One study [10] on abdominal aortic aneurysms in rats used the same elastase product as the one used in our study. In this study, high concentrations of elastase-induced unexpected vascular damages. This study suggests that mediated inflammatory proteins may have been included as contaminants inducing excessive inflammation, and were derived from the porcine pancreas when the elastase was first extracted [10]. Therefore, the correct concentration and incubating time for the respective product have to be considered when creating aneurysm models. In this study, elastase created huge aneurysms; however, by simultaneously inducing sterile abscesses in the aneurysmal sac, this model became unsuitable as an aneurysm animal model.
Enzymatically induced SA undergoes inflammation and inflammatory cells are often found in the walls of the aneurysm [34]. Elastase induces inflammation and apoptosis which results in the vascular wall to weaken before an aneurysm is formed [6, 34, 35]. The mechanism of elastase-induced aneurysms is not fully known and the mechanism of papain-induced aneurysms is completely unknown. Elastase is derived from an animal product and papain from a plant product. Generally, animal-derived substances are xenogenic, not stable, and likely to cause immune reactions. Although elastase and papain are both proteolytic enzymes, their aneurysm-inducing mechanisms may be different because they are derived from a different source. We compared papain with elastase using the concentration unit “mg”; however, an additional comparison using another measurement, such as “unit (U)”, will be needed. We used a concentration of 1 mg based on a report by de Oliveira et al.[16], but many studies using elastase chose “unit” as measurement unit [10, 30, 33, 36]. While using “mg” as the standard unit, we found histopathologic differences between papain and elastase.
A few concerns and limitations exist in this study. First, not all studies use the same product and concentration of elastase; each study uses a different product from a different company. Even if earlier studies used the same concentration, different products can have different proteolytic powers. Also, the concentrations of elastase used in different studies on rabbits were 20 U [30, 31], 100 U [33], and 200 U/mL [37]. Second, the individual number of rabbits in each group was too small. To achieve higher significance, an additional large scale study should be performed. Moreover, further studies on ideal concentrations and incubating times for products with papain and elastase are also needed. When treated with 1 mg of papain and elastase each, papain-induced aneurysms were more stable than elastase-induced aneurysms. Inducing inflammation is an essential process in creating an aneurysm model; however, excessive inflammation can lead to the development of sterile abscesses in aneurysms, rendering the resulting model unsuitable for studying SA. Papain is therefore our candidate for enzymatic aneurysm models in rabbits. Further studies testing varying conditions such as concentration and incubation time are needed to find the most suitable procedure for the creation of papain-induced aneurysm animal models.
