Introduction
Among cancers that affect women, breast cancer is of enormous concern worldwide [1]. Age, sex, hormonal factors, and family history can be common causes for breast cancer. The treatment of early breast cancer includes treating the disease locally with surgery, radiation therapy, or both and treating microscopic systemic disease with either one or a combination of chemotherapy, endocrine therapy, or biologic therapy. Adequate treatment, suitably timed, is associated with a higher survival rate.
Doxorubicin (DOX), an anthracycline antitumor drug, is primarily prescribed for chemotherapy. DOX kills tumor cells by inducing DNA damage [2], working as a topoisomerase inhibitor [3, 4], and producing reactive oxygen species (ROS) [5]. However, DOX usage is a double-edged sword, as it affects not only tumor cells but also normal cells in a dose-dependent manner. Thus, cardiotoxicity is a well-known side effect of DOX [6]. Additionally, numerous studies reported that DOX induces apoptosis in cardiomyocytes in vitro [7-9]. On the other hand, the cardio-protective effect of estrogen is, likewise, well studies. Estrogen interacts with many receptors involved in calcium ion homeostasis, anti-apoptotic effects, and mitochondrial metabolism, thereby reducing the risk of cardiac diseases [10, 11].
As mentioned previously, patients are commonly administered a combination treatment of endocrinal and chemical therapy. The hormonal treatment uses selective estrogen receptor modulators, selective estrogen receptor down regulators, and aromatase inhibitors, which lower estrogen levels [12, 13]. We hypothesized that decreased estrogen levels may alter defensive mechanisms of the heart, leading to DOX-induced cardiotoxicity. In this study, the mice were divided into a control group, ovariectomized (OVX) group, DOX-treated group, and OVX plus DOX-treated group. We explored the relationship between absence of estrogen and DOX-induced cardiotoxicity by screening genes and cytokine levels of the mouse heart.
Materials and Methods
Primary antibodies used were; Cleaved caspase-3 (9661S), Alpha tubulin (66031-1-Ig) Secondary antibodies used were; Mouse HRP secondary antibody (115-035- 174), Rabbit HRP secondary antibody (211-032-171)
C57BL/6 WT female mice were housed in the pathogen- free facility at Chungnam National University under a standard 12 h light:12 h dark cycle and fed with standard chow with water provided ad libitum. All mouse experiments were approved and performed in accordance with the Chungnam National University Animal Care Committee. Three-month-old female C57BL/6 mice (n = 19) were used in this study. The mice were anesthetized (inhalation of isoflurane manually) under aseptic conditions and were randomly subjected to ovariectomy (OVX group; n = 9). The OVX was performed with a small incision along the ventral abdominal midline. The ovaries were bilaterally clamped and removed through the incision. The uterine horns were tied, and the uterus was left intact. 2 weeks after the operation, both control and OVX mice separated into two new groups according to DOX treatment (total 4 group: control (Con) (n=3), ovariectomy (OVX)(n=5), DOX treatment (DOX)(n=7), and doxorubicin and ovariectomy treatment(DOX+OVX) (n=4)). DOX (25 mg/kg/day) was exposed into mouse by intraperitoneal (i.p.) treatment every day for 5 days. Necropsy was conducted after last administration, and hearts were isolated.
Total RNA extracts from mouse heart were prepared using the TRIzolⓇ Reagent (Thermo Fisher Scientific,Walthm, MA). Reverse transcription was performed with 1.5 μg of total RNA and Excel RT Reverse transcriptase kit (RP1300, SMOBIO, Hsinchu City, Taiwan) following manufacturer’s protocol. Quantitative PCR (real-time PCR) was carried out using specific primers (Table 1), Excel Taq Q-PCR Master Mix (TQ1101, SMOBIO) and Stratagene Mx3000p (Agilent, Santa Clara, CA) equipped with a 96-well optical reaction plate. All experiments were run in triplicate, and mRNA values were calculated based on the cycle threshold and monitored for melting curve.
Heart samples were homogenized by protein lysis buffer [T-PER (#78510, Thermo)] containing proteinase inhibitor (PMSF). Homogenized samples were quantified by bradford assay with PRO-Measure solution (Intron, #21011) and proceeded to protein electrophoresis (SDSPAGE) after 5 min of boiling in 100°C. Gels were blotted by wet transfer with Bio-Rad Power Pac in 350 mA. Membranes were blocked for 1 h in skim milk and incubated with a primary antibody (see above) for overnight at 4°C. After 3 times of washing with PBS-T or TBS-T, membranes were incubated with secondary antibodies (AE-1475 anti-rabbit, Bioss, Aachen, Germany) for 2 h diluted with 1:10000 in skim milk or BSA. Results were detected with ECL solution (XLS025-0000, Cyanagen, Bologna, Italia) and Chemi Doc (Fusion Solo, Vilber Lourmat).
Results
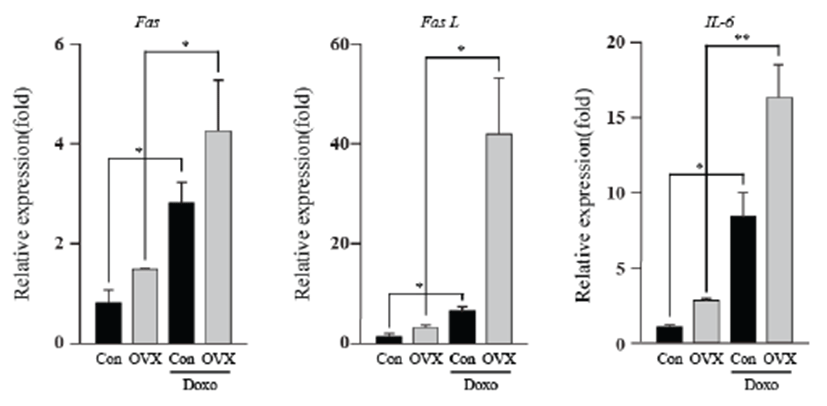
Although the cardiotoxicity of DOX is well established, we need to gain insight into how the absence of estrogen might affect the cardiotoxicity induced by doxorubicin. Thus, we screened mRNA levels of apoptotic genes, energy-related genes, and IL-6 in the mouse heart by qPCR. Interestingly, female mice showed substantially increased in Fas, Fas L and IL-6 mRNA after ovariectomy. As shown in Fig. 1, doxorubicin-treated groups showed significantly higher expression of Fas (3.52 fold, con vs DOX; 2.89 fold, OVX vs OVX+DOX; P<0.05), Fas L (5.32 fold, con vs DOX; 13.31 fold, OVX vs OVX+DOX; P<0.05), and IL-6 (8.29 fold, con vs DOX; 5.91 fold OVX vs OVX+DOX; P<0.05, P<0.01) than that of the control group. The OVX plus DOX-treated group showed the highest expression of these genes compared to that of the other groups. This result indicates that combined treatment of OVX and DOX induced heart damage due to the Fas-related extrinsic apoptosis pathway and inflammation.Fig. 2Fig. 3
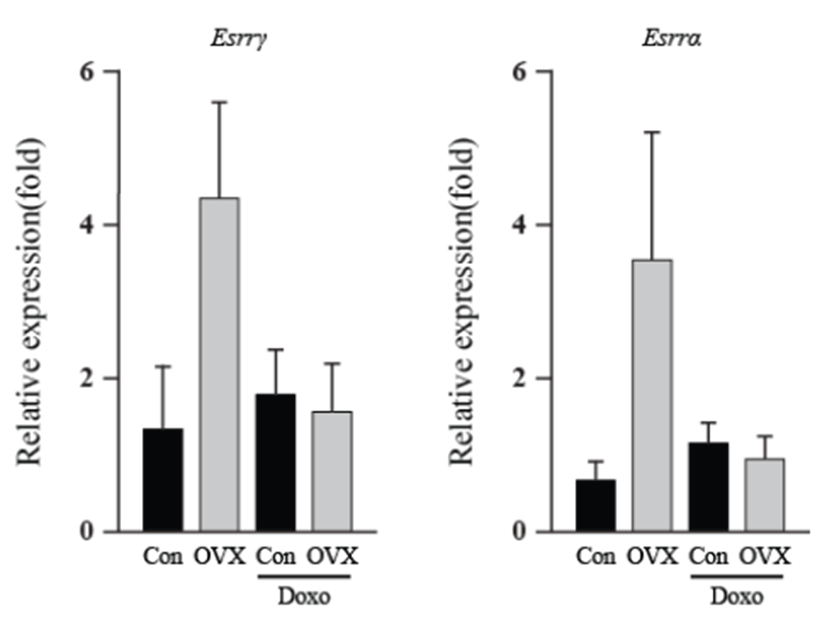
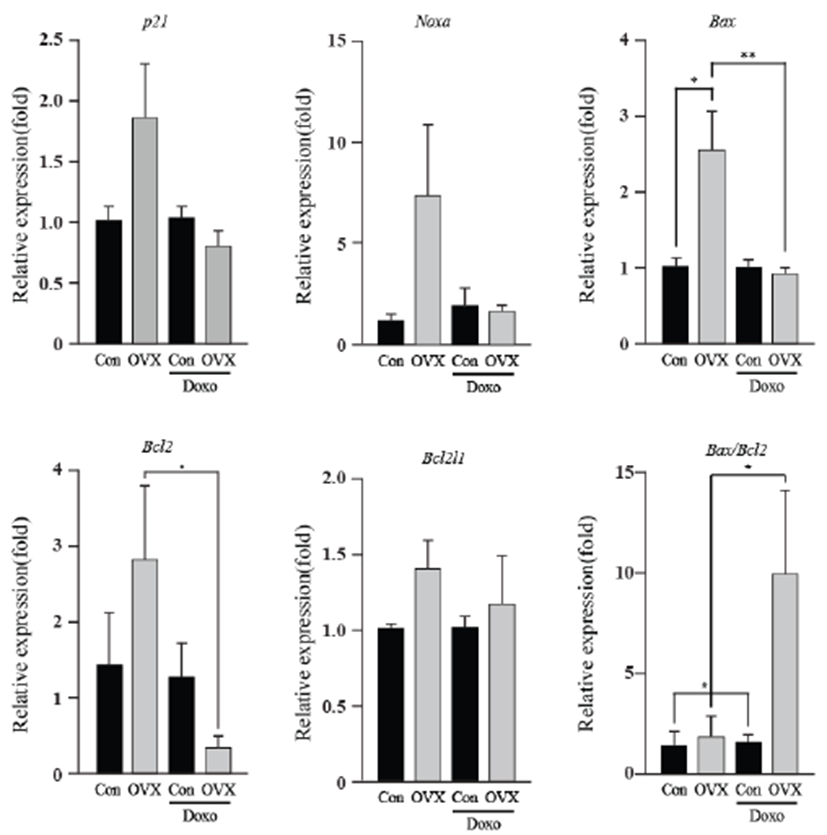
As an energy metabolic regulator in the heart, the estrogen- related receptor γ (Esrrγ) is a far more significant indicator than the estrogen related receptor α (Esrrα). Apoptotic genes showed little alteration in expression, excluding Bcl2 and the Bax/Bcl2 ratio. The energy-related genes (Esrrγ and Esrrα) and apoptotic genes (p21, Noxa, Bax, Bcl2l1 and Bcl2) showed similar expression, and the highest expression was found in the OVX group. The Bcl2 levels of the OVX group were higher than those of the OVX plus DOX-treated group (8.50 fold, P<0.05). The Bax/Bcl2 ratio increased in the OVX plus DOXtreated group (6.44 fold, P<0.05) compared to that in the OVX group. These results suggest that OVX activates the intrinsic apoptosis pathway, and this was evident in the OVX plus DOX-treated group.
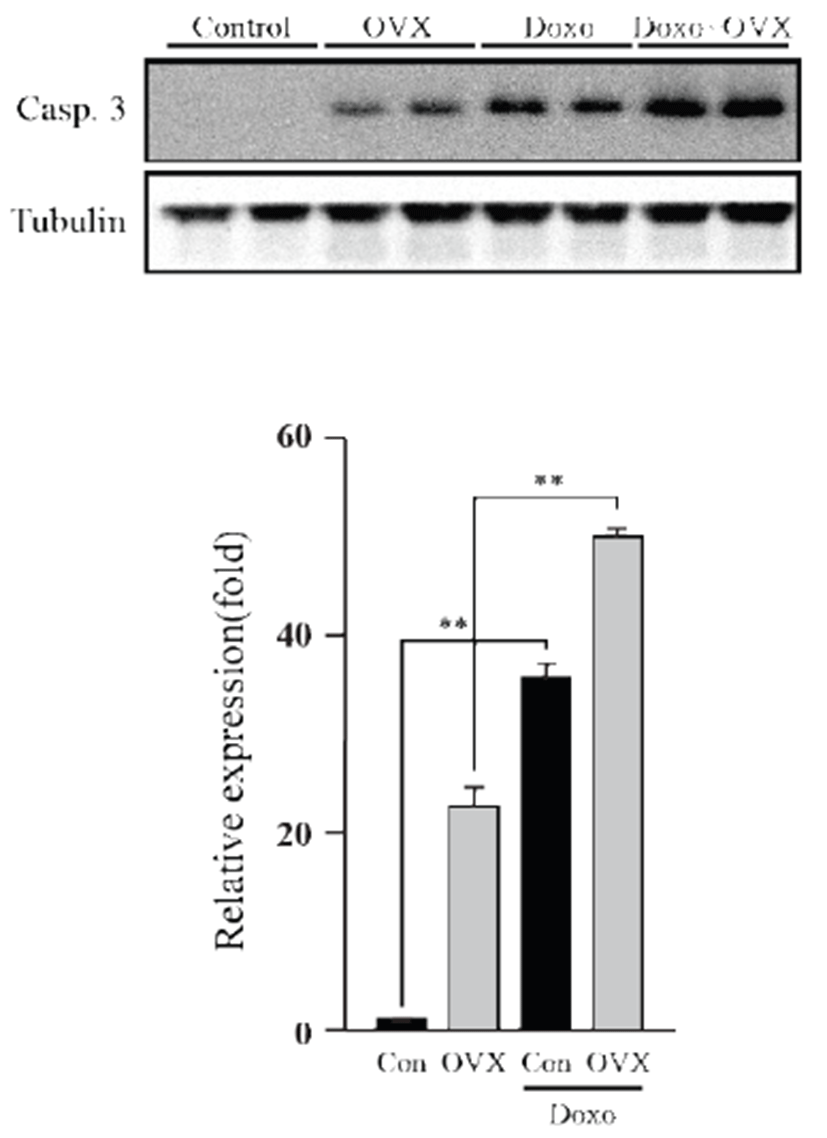
Apoptosis was evaluated by western blot, using caspase- 3. As shown in the figure 4, we observed increase (P<0.01) of caspase-3 in OVX group compared to control group. Caspase-3 expression of the DOX-treated group was higher than that of the control group (35.36 fold, P<0.01), whereas the OVX groups similarly showed increased expression (1.41 fold, P<0.01). These data suggest that DOX induces apoptosis in the mouse heart, which is significantly increased under a female hormone deficiency.
Discussion
The clinical use and efficacy of DOX is severely hampered by its cardiotoxicity. Hormonal therapy disposes breast cancer patients toward low estrogen levels. As this hormone positively affects the cardiovascular system, we hypothesized that in conjunction with a lowered estrogen state, DOX treatment results in elevated negative effects.
To observe DOX-induced cardiotoxicity, western blotting was performed to confirm apoptosis through its representative marker, caspase-3. We found that caspase-3 significantly increased in the test groups except vehicle control. In addition, a previous study suggested that DOX-induced cardiotoxicity causes apoptosis of heart cells [14]. Moreover, it is well established that loss of endogenous estrogen was linked with heart failure because of cardiomyocyte apoptosis [15]. The present data suggest that DOX exposure within heart acts to enhance the apoptosis, especially under condition where the supply of estrogen is limited. Following western blot analysis, we screened certain genes involved in apoptosis and those related to cardiac damage at the mRNA level by using qPCR. The Fas and Fas ligand showed increased expression, implying that it stimulated the extrinsic apoptosis pathway [16]. According to these results, this apoptosis pathway was stimulated by estrogen deficiency. Expression of interleukin-6, a prominent pro-inflammatory cytokine, was significantly increased. This suggests that loss of estrogen leads to elevated inflammation, corroborating with the results of an earlier report suggesting that DOX causes inflammation in the heart [17]. In a recent study, we found that inflammatory response can sequester sulfate conjugates from the endogenous steroid hormones and may suppress binding of sex steroid hormones to their receptors in the whole body [18]. Noxa, p21, Bax, Bcl2, and Bcal2l1 are genes involved in the intrinsic apoptosis pathway. There were insignificant changes in their expression, except Bcl2, an anti-apoptotic protein, which showed a significant decrease. Although Bax expression itself was unsupportive of our theory, the Bax/ Bcl2 ratio showed that the absence of estrogen affects DOX-induced apoptosis. Additionally, this validates previous knowledge that DOX-induced cardiotoxicity stimulates the intrinsic apoptosis pathway [19]. Esrrγ and Esrrα are involved in energy metabolism and homeostasis in the heart [20]. An earlier report suggests Esrrγ and Esrrα are upregulated by estrogen [21]; however, our study did not show adequate information supporting this.
Thus, evidently, lowered levels of estrogen together with DOX induce cardiotoxicity in the heart. Thus, combination treatment of hormonal and DOX therapy for breast cancer patients must be prescribed with requisite precautions. Moreover, DOX treatment for menopausal women with low estrogen levels or women with low estrogen levels due to other reasons requires contemplation. On the other hand, further studies are required to establish whether administration of estrogen could help hormone receptor-negative breast cancer patients in light of its cardioprotective function.