Helicobacter pylori infection induces inflammation in gastric epithelial cells; this may play a significant role in the pathological events associated with infection by this organism [1, 2]. H. pylori induces inflammatory associated gene expression in gastric epithelial cells, including that for reactive oxygen species (ROS), cyclooxygenase- 2 (COX-2), and IL-8 [3, 4]. Among the cytokines induced in the gastric mucosa colonized by H. pylori, IL-8 is one of the major proinflammatory cytokines, first isolated from monocytes as a neutrophil chemoattractant [5]. IL-8 plays a crucial role in the initiation and maintenance of inflammatory response and recently has been identified to function as proangiogenic or carcinogenic factor based on the findings that gastric cancer cells in surgical specimens overexpressed IL-8compared with corresponding normal mucosa [5].
Tribulus terrestris L. is an annual creeping herb of the family Zygophyllaceae. T. terrestris was known as puncture vine or caltrop. It is 30 to 70 cm high and has pinnate leaves and yellow flowers. It is widely growing in tropical and moderate regions of the world, including Africa, Western Asia, China, Japan, Korea, and southern Europe [6]. It has been used since ancient times in folk medicine for treating diuretic, hypertension, edema, eye problems, sexual impotency, inflammation, and rheumatoid arthritis [7-10].
The goal of this study was to determine the anti-inflammatory effects of the fruits of Tribulus terrestris L. extract (TTE) against experimental H. pylori infection.
AGS cells (ATCC CRL 1739; human gastric adenocar- cinoma epithelial cell line) were cultured in RPMI (Sigma) supplemented with 10% de-complement fetal bovine serum (Invitrogen). Penicillin and streptomycin (GIBCO BRL) were also added if needed.
H. pylori (ATCC49503) was obtained from American Type Culture Collection (Manassas, VA, USA), and cultured on brain heart infusion (BHI) broth in an anaerobic chamber with 10% CO2, 5% O2, and 85% N2 at 37°C with enough humidity.
The dry mass of the fruits of Tribulus terrestris was purchased from an Oriental Pharmacy (Iksan, Korea). The procedure for preparing TTE was as follows. The air-dried mass of the fruits of Tribulus terrestris (100 g) was cut into pieces and extracted twice with 50% (v/v) ethanol (Six times as much as the weight of the dried plants) for 3 hrs at 80°C. After filtration through a 400- mesh filter cloth, the filtrate was refiltered through filter paper (Whatman, No. 5) and concentrated on a rotary evaporator (EYELA, Tokyo, Japan), and the concentrated filtrate was evaporated to dryness under vacuum with freezing dryer (Labconco, USA). Finally, the solid residue was collected, placed in sealed bottles, and stored at -20°C. The extract yield of the fruits of Tribulus terrestris with 50% ethanol was 20.60%. Protodioscin, which was used for the standard material of TFE composition, was purchased from Sigma Aldrich (USA). The Protodioscin composition of TTE was analyzed by LC. Waters ACQUITY LC system (Waters Corp., Milford, USA) was used for LC system. The column was C18 type ACQUITY UPLC BEH (2.1 × 50 mm, 1.7 μM, Waters Corp., Milford, USA). A Waters Nova Pack C-18 column (ACQUITY UPLC BEH (2.1 × 50 mm, 1.7 μM, Waters Corp., Milford, USA)) was employed. The wavelength of the UV detector was set at 300 nm. The column temperature was set at 30°C with a flow rate of mobile phase at 0.6 mL/min (0.1% H3PO4 /acetonnitrile).
Cell viability was determined by the tetrazolium salt reduction method using WST-1 according to the manufacturer's instructions (BioVision, Mountain View, CA, USA). In brief, 2 × 104 cells/well were plated in 96-well plates and cultured overnight. The cells were incubated with/without TTE for 24 hrs, after which 10 mL of WST- 1 was added, and the cells were incubated for an additional 1 hr. The number of viable cells was estimated by measuring the absorbance at 450 nm. All treatments were performed in quadruplicate, and the experiment was repeated three times. Cell viability was calculated as the relative absorbance compared with that of control cultures.
AGS cells (2 × 104 cells) were seeded in 96-well plates. The cells were pretreated with TTE (12.5, 25, 50 and 100 mg/mL) for 24 hrs. The cells were then infected with H. pylori for 24 hrs. The culture medium was collected, and IL-8 measurements were performed using ELISA kits (Enzo Life Sciences, Farmingdale, NY, USA). Each sample was tested in triplicate.
The extract yield of the fruits of Tribulus terrestris with 50% ethanol was 20.60%. We analyzed TTE composition by LC. The concentration of Protodioscin in TFE was 310 μg/mL.
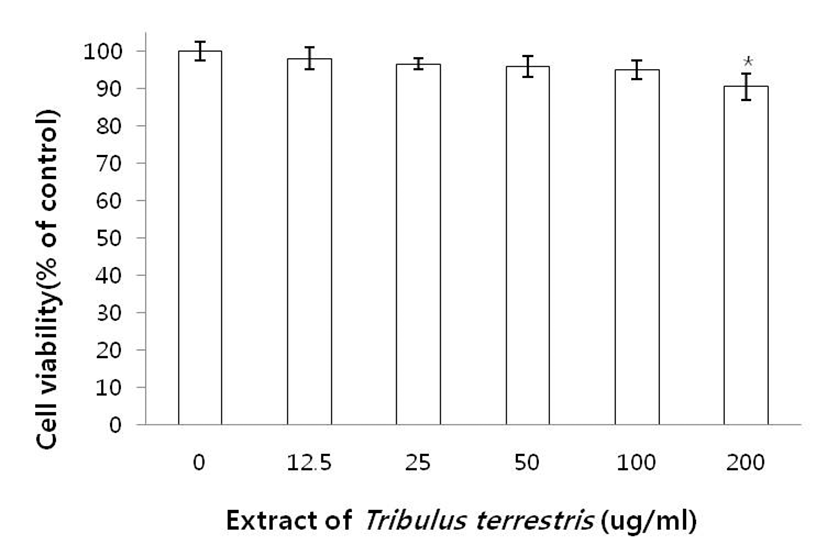
It was confirmed that exposure to 100 μg/mL of TTE had no significant effect on cell proliferation at the concentrations examined (12.5–200 μg/mL) (Fig. 1). It was therefore concluded that TTE at these concentrations had no cytotoxic effects on AGS cells and could be used in this study.
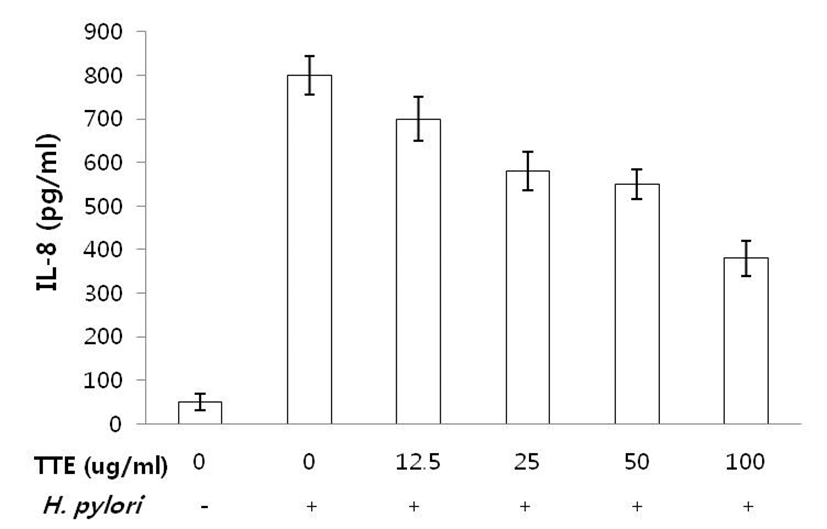
IL-8 production in gastric epithelial cells infected with H. pylori with or without pre-incubation of TTE from 12.5 to 100 μg/mL was measured. Pretreatment of H. pylori- infected AGS cells with 12.5, 25, 50, and 100 μg /mL of TTE for 24 hrs significantly decreased IL-8 production by 12.5%, 25%, 27.5%, and 50%, respectively, compared to H. pylori-infected cells without TTE (Fig. 2).
H. pylori highly colonises the human gastric mucosa and perfectly adapted to that environment. Concerning pathology, H. pylori causes gastritis and is classified as a major primary risk factor for the development of gastric or peptic ulcers, gastric adenocarcinoma, and mucosaassociated lymphoid tissue lymphoma [11].
In this study, we did not evaluate the effect of TTE on the H. pylori-induced ROS and COX-2. However, IL‑8 is a chemoattractant factor for neutrophil recruitment and a critical immune mediator for the pathogenesis of chronic gastritis caused by H. pylori infection. In addition, various studies have reported that high expression of IL‑8 is correlated with poor prognosis of gastric cancers or gastrointestinal tumorigenesis, including angiogenesis [12]. Therefore, IL‑8 has been suggested as a therapeutic target in gastric cancer. The present study revealed that in vitro pre‑treatment and co‑treatment of TTE effectively inhibited H. pylori‑induced production of IL‑8 in gastric epithelial cells, suggesting that TTE may have preventive as well as therapeutic effects on H. pylori‑mediated inflammation.
T. terrestris is used in folk medicine as tonic, aphrodisiac, analgesic, astringent, stomachic, anti-hypertensive, diuretic, lithon-triptic, and urinary anti-infective [13-16]. The main components of T. terrestris are saponins, diosgenins, alkaloids, and amides [17, 18]. The ethanol extract of T. terrestris inhibited the growth and acid production of Streptococcus mutans [19]. Other studies have shown that this extract has a good antibacterial activity against gram positive bacteria such as Staphylococcus aureus and Enterococcus faecalis and gram negative bacteria such as E. coli. It indicates that there is a broad spectrum of antibiotic compounds or simply general metabolic toxins in the fruits of Tribulus terrestris L. (Tribuli Fructus) extract [20].
In this study, we found that TTE inhibited H. pylori‑induced IL‑8 secretion, thus augmenting their benefit in regard to protection of gastric epithelial cells. This study suggests that ingestion of these plant extracts could have therapeutic implications for patients with H. pylori induced gastritis and duodenal ulcer.