Introduction
Mutations in Ras were the first specific genetic alterations identified in human cancer [1]. Intensive studies have reported that gain-of-function mutations in Ras are found in approximately 30% of all human cancers. Ras proteins are critical regulators that link diverse extracellular stimuli, including peptide growth factors, cytokines, and hormones, with a diverse range of biological responses [2, 3]. Ras proteins cycle between guanosine- 5′-triphosphate (GTP)-bound active forms and guanosine diphosphate-bound inactive forms. This exchange is mediated by guanine nucleotide exchange factors or GTPase-activating proteins (GAPs) [4]. Ninety-eight percent of Ras mutations occur at one of the amino acid residues G12, G13, or Q61. The single amino acid substitution at 12, 13, or 61 creates mutant proteins that are insensitive to GAP action [5], resulting in uncontrolled cell proliferation.
The four Ras proteins—H-Ras, N-Ras, K-Ras 4A, and K-Ras 4B—are localized to the cytoplasmic face of the plasma membrane through a series of posttranslational processing [6]. Farnesyl transferase catalyzes the addition of a C15 farnesyl isoprenoid lipid onto cysteine in the Ras C-terminal CAAX motif (where C is cysteine, A is an aliphatic compound, and X is typically methionine or serine). Inhibition of farnesylation using farnesyl transferase inhibitors (FTIs) disrupts the association with the Ras membrane and aberrant Ras activity to transform the cells [7, 8]. Although FTIs have been developed to inhibit oncogenic Ras-transformed cells and suppress the growth of carcinoma xenografts, their cytotoxicity against normal cells has been controversial. For example, manumycin, an analog of farnesyl diphosphate, inhibits the proliferation of Chinese hamster ovary (CHO) cells that express insulin receptors [9]. By contrast, James et al. (1994) demonstrated that BZA-5B, another FTI, ap- pears to be relatively nontoxic because it does not inhibit the proliferation of untransformed cells [10]. These results are explained by a bypass mechanism through which K-Ras and N-Ras can act as a substrate for geranylgeranyl transferase-1 in the presence of FTIs [11].
Insulin controls many aspects of metabolism, growth, and survival. The major insulin signaling pathways regulate metabolism and gene expression, with central roles for phosphatidylinositol 3-kinase/protein kinase B and Ras/Raf/MEK [12-15]. Ras protein plays a central role in the insulin and insulin-like growth factor-1 signaling pathways and is required for gene expression and DNA synthesis [16]. Recently, we found that lonafarnib partially inhibits DNA synthesis stimulated by insulin, but not glucose uptake in 3T3-L1 adipocytes [17]. On the contrary, manumycin suppresses the antiapoptotic action of insulin in untransformed CHO cells [9]; therefore, it is important to determine whether FTIs affect normal cellular functions, such as insulin actions.
In this study, we investigated the effects of farnesyl transferase inhibitor YH3096 and its derivatives YH3938 and YH3945 on the mitogenic and metabolic actions of insulin. We demonstrated that YH3096 blocked farnesylation of H-Ras but did not affect processing of K-Ras and N-Ras. YH3096 inhibited insulin-mediated DNA synthesis but did not affect c-Jun expression or membrane ruffling induced by insulin. In addition, YH3096 and its derivatives did not block insulin-induced glucose uptake.
Materials and Methods
The farnesyl transferase inhibitors —YH3096, YH3938 and YH3945—were kindly provided by Yuhan Corporation (Seoul, South Korea). Cys-Ile-Ile-Met motif was utilized as a template peptide for synthesis of YH3096 and its derivatives [18]. Pan-Ras monoclonal antibodies were purchased from Merck Millipore (Billerica, USA) and anti-H-Ras antibody and anti-c-Jun antibody were from BD biosciences (San Diego, USA). Methyl-[3H]-thymidine, and 2-deoxy-D-1-[3H] glucose were obtained from GE healthcare (Pittsburg, USA). TRITC-conjugated phalloidin was purchased from Jackson Immunoresearch Laboratories (West Grove, USA). All culture media were from Gibco (Waltham, USA). All other reagents were purchased from Sigma (St. Louis, USA).
HIRc-B cells, which are rat-1 fibroblasts overexpressing the human insulin receptors, were maintained as previously described [16]. To investigate insulin actions, HIRc-B cells were starved in DMEM supplemented with 4 mM L-glutamine and 100 unit/mL penicillin-100 μg/ mL streptomycin for 24 h. 3T3-L1 preadipocytes were maintained as fibroblasts and differentiated as described [19].
HIRc-B cells were grown in 12-well plates and treated with the indicated doses of YH3096 for 24 h. Cells were washed and lysed, and cell lysates were analyzed on 12.5% SDS-PAGE followed by immunoblotting with anti-Ras antibodies.
HIRc-B cells were grown in 24-well plates and serumstarved in the presence of dimethyl sulfoxide (DMSO) or 0.1, 0.5, 1 μM YH3096 for 24 h. Then cells were stimulated with insulin of the indicated concentrations for 16 h and then pulsed with [3H]-thymidine, 0.5 μCi/mL, for 4 h at 37°C. The cells were washed, and the incorporated thymidine was counted [20].
To investigate the effect of YH3096 on membrane ruffling [16], HIRc-B cells were grown on 12-mm glass coverslips and incubated with serum-free DMEM in the presence of YH3096 (0.5 or 1 μM) for 24 h. Then cells were stimulated with insulin (100 ng/mL) for 10 min. The cells were fixed, permeabilized, and incubated with TRITC-conjugated phalloidin (0.1 mg/mL) for 1 h. Results were analyzed on the fluorescence microscopy. The results represent the mean of at least three independent experiments in which at least 300 cells were counted. HIRc-B cells were grown on 12-well plates, and the medium was replaced with serum-free DMEM in the presence of YH3096 for 24 h. Then cells were stimulated with insulin (100 ng/mL) for 4 h and immunoblotted with anti-c-Jun antibody.
The differentiated 3T3-L1 adipocytes were incubated with the indicated doses of YH3096 for 4 d, and then glucose uptake was examined as previously reported [19].
Results and Discussion
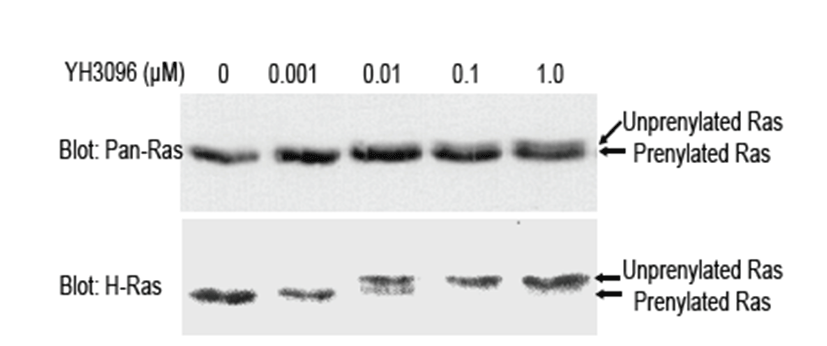
The farnesyl transferase inhibitors —YH3096, YH3938 and YH3945—are developed by Yuhan Corporation (Seoul, South Korea) by utilizing Ras CAAX motif as a template peptide [18]. It was shown that YH3096 and its derivatives blocked the cell growth by inhibiting FTase, leading to G2/M enrichment in human tumor cells harboring ras mutation [18]. To examine the effects of YH3096 on insulin actions, we used insulin-responsive HIRc-B cells derived from normal rat-1 fibroblasts and engineered to express 100,000 human insulin receptors per cell [21]. Because all four Ras proteins are expressed in HIRc-B cells and well-known substrates for FTase, we first attempted to check the effect of YH3096 on Ras prenylation in HIRc-B cells. To do this, HIRc-B cells were treated with the indicated amounts of YH3096 for 24 h. Because a prenylated Ras protein migrates faster on sodium dodecyl sulfate polyacrylamide gel electrophoresis than its unprenylated counterpart, we performed an immunoblot using antibodies against pan-Ras and H-Ras to differentiate the prenylated Ras protein from the unprenylated form. As shown in Fig. 1, immunoblot analysis with anti-Pan-Ras antibody showed that the endogenous H-, K-, and N-Ras proteins in DMSO-treated HIRc-B cells are normally prenylated. In contrast, treatment with 1.0 μM YH3096 rendered approximately 30% of endogenous Ras unprenylated. Subsequently, an immunoblot using H-Ras antibody revealed that >95.0% H-Ras was unprenylated by 1.0 μM of YH3096. Consistent with previous reports [8], our results demonstrated that prenylation of exclusively farnesylated H-Ras is completely inhibited by YH3096, but that K-Ras and N-Ras become alternatively genranylgeranylated in the presence of YH3096.
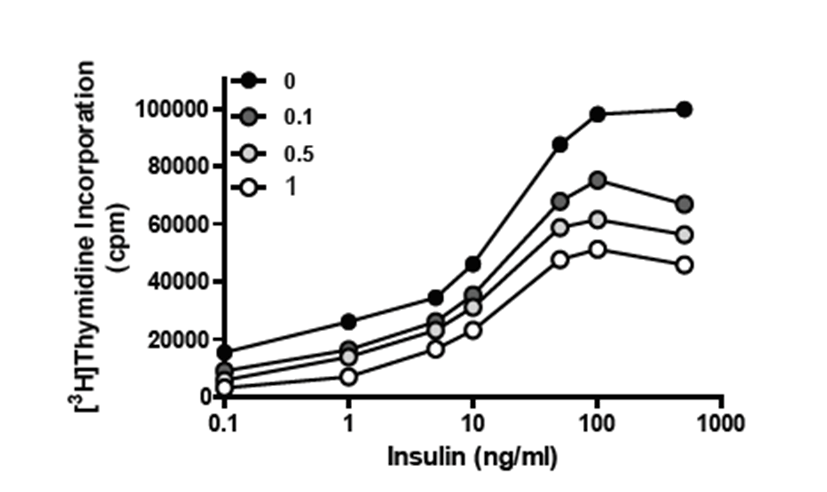
Knowing that Ras protein is important for insulin-induced DNA synthesis [16], we next investigated the effects of YH3096 on DNA synthesis after insulin stimulation. HIRc-B cells were starved in the presence or absence of 0.1, 0.5, or 1.0 μM YH3096 for 24 h. We then measured the amount of radiolabeled thymidine incorporated into the DNA in response to the increasing concentrations of insulin. As shown in Fig. 2, insulin treatment stimulates DNA synthesis up to fivefold in a dose-dependent manner; however, treatment with YH3096 gradually inhibits insulin-induced DNA synthesis. This result suggests that farnesylation of Ras protein is largely implicated in DNA synthesis, although geranylgeranylation of K-Ras and N-Ras might compensate for the loss of farnesylated Ras function to a lesser extent.
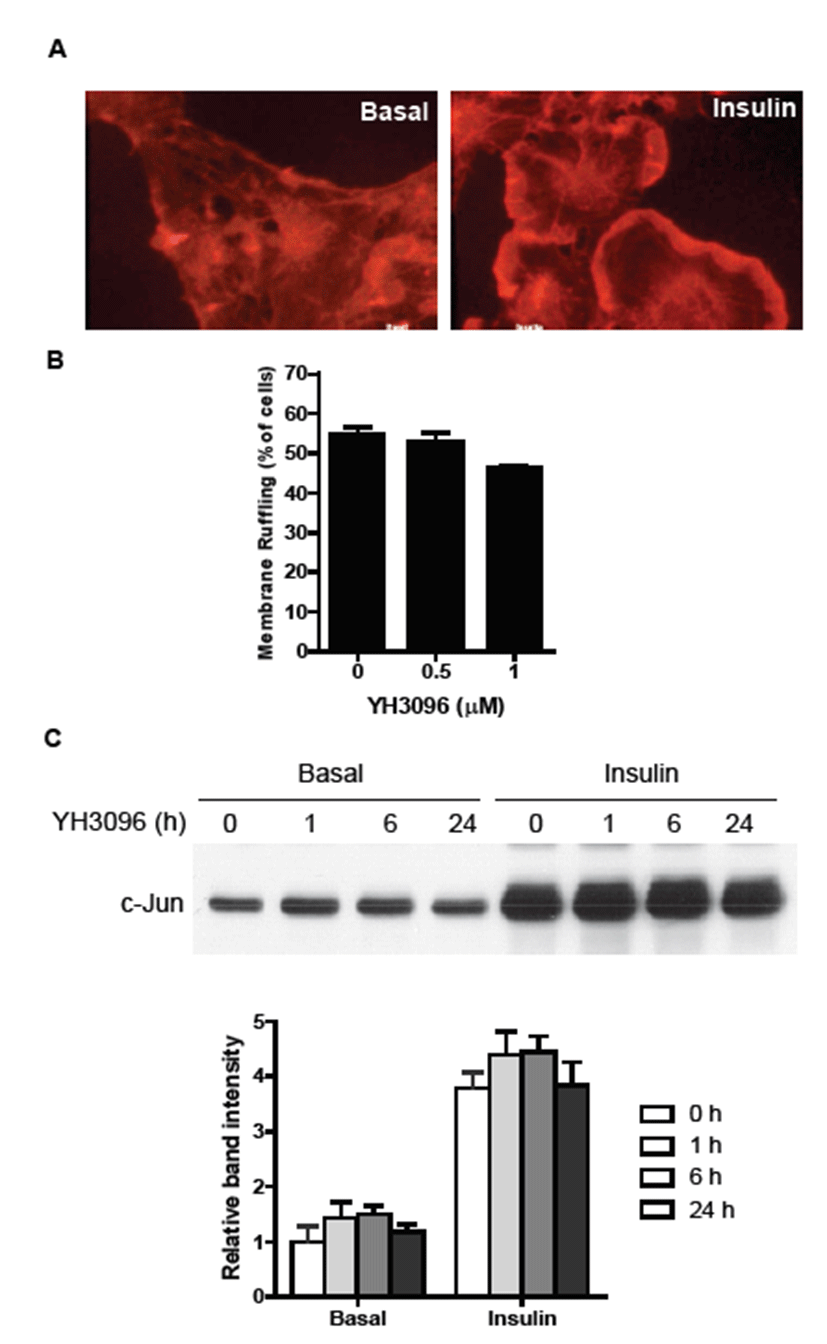
To determine whether YH3096 affects other insulin actions, including membrane ruffling, c-Jun expression, and glucose uptake, serum-starved HIRc-B cells were treated with 0.5 or 1.0 μM YH3096 for 24 h and then stimulated with 100 ng/mL insulin. We first determined the effect of YH3096 on insulin-mediated membrane ruffling. Insulin stimulation of HIRc-B cells rapidly induced membrane ruffling (Fig. 3A), but treatment with YH3096 had no effect on it (Fig. 3B). Similarly, YH3096 had no effect on insulin-mediated c-Jun expression (Fig. 3C).
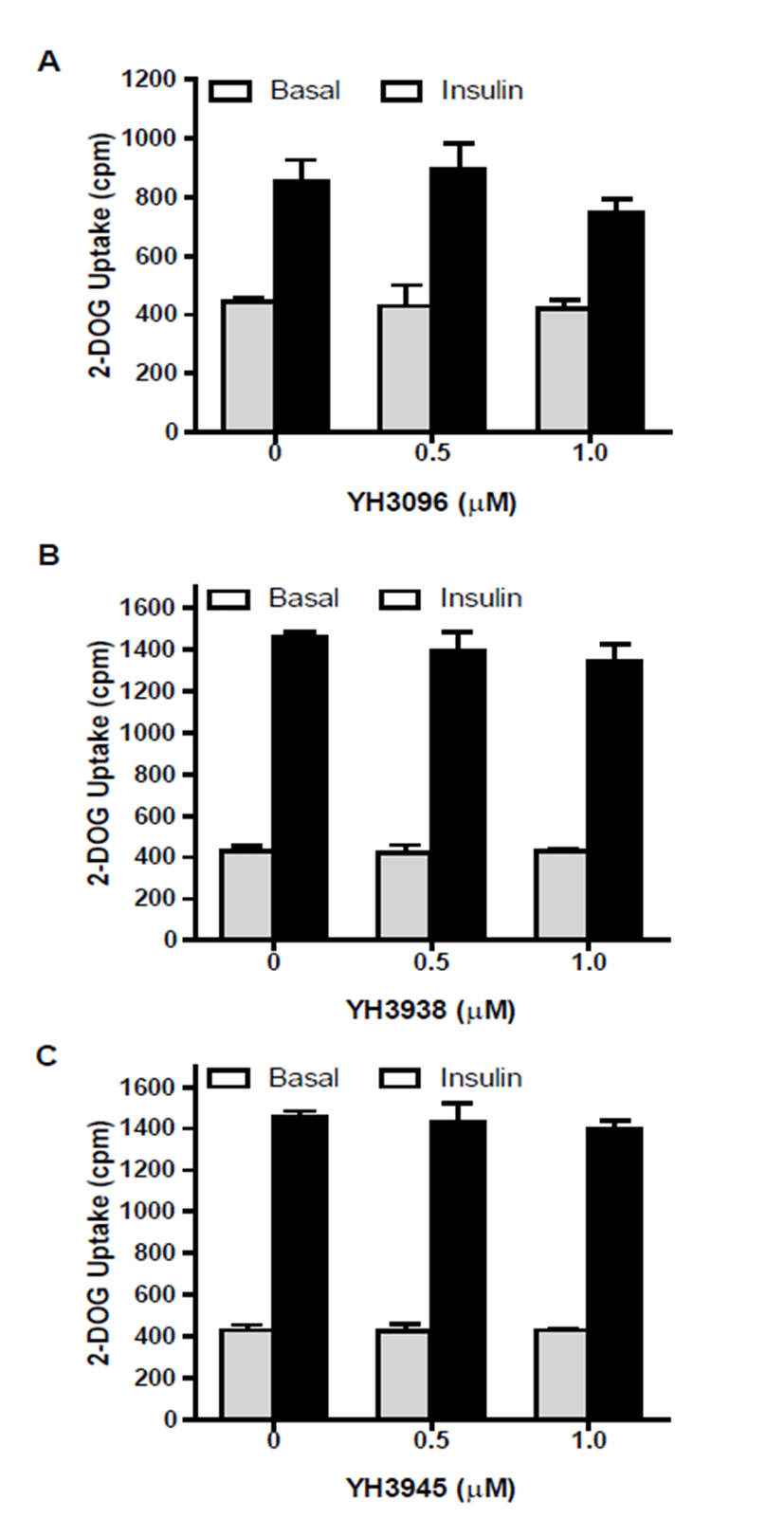
To determine whether YH3096 modulates insulin-mediated glucose uptake, differentiated 3T3-L1 adipocytes were treated with either 0.5 or 1.0 μM YH3096 for 4 d and subjected to an assay of 2-deoxyglucose uptake. As shown in Fig. 4A, insulin similarly increases glucose uptake in both DMSO- and YH3096-treated 3T3-L1 adipocytes. In addition, YH3096 derivatives YH3938 and YH3945 had no effect on insulin- induced 2-deoxyglucose uptake by insulin (Figs. 4B and 4C).
Interestingly, insulin’s metabolic pathway, unlike the mitogenic pathway, was not affected by YH3096. These results indicate that geranylgeranylation of K-Ras and NRas proteins is mainly involved in membrane ruffling, c-Jun expression, and glucose uptake. It will be important to elucidate the differential functions of farnesylated and geranylgeranylated Ras. Our study demonstrates that YH3096 has an inhibitory effect on insulin-induced DNA synthesis by blocking Ras farnesylation.