Original Article
Effect of Rubus coreanus leaf and stem extract on boar spermatozoa
Young-Joo Yi1
,*

, Min Cho1, Jung Min Heo2, Sang-Myeong Lee1
1Division of Biotechnology, Safety, Environment and Life Science Institute, College of Environmental and Bioresource Sciences, Chonbuk National University, Iksan 54596, Korea
2Department of Animal Science and Biotechnology, Chungnam National University, Daejeon 34134, Korea
*Corresponding author: Young-Joo Yi Division of Biotechnology, Safety, Environment and Life Science Institute, College of Environmental and Bioresource Sciences, Chonbuk National University, Iksan 54596, Korea +82-63-850-0835, +82-63-850-0834
yiyj@jbnu.ac.kr
© Research Institute of Veterinary Medicine, Chungbuk National University. All rights reserved.
Received: Apr 25, 2017; Revised: Jun 19, 2017; Accepted: Jun 23, 2017
Abstract
Rubus coreanus is known to have diverse biological properties, such as free radical scavenging activity and antibacterial activity. In the present study, Rubus coreanus leaf and stem extract (RLSE) was used in boar semen preservation whether it has a beneficial effect on assisted reproductive technology (ART) in mammals. Boar spermatozoa were preserved in Beltsville thawing solution (BTS) in the presence of varying concentrations of RLSE (0-10 μg/mL). Sperm motility, sperm viability, and intracellular reactive oxygen species (ROS) levels were examined after 2 days of preservation. The percentage of total motile spermatozoa and progressive motile spermatozoa improved in the spermatozoa preserved with 0.5 μg/mL RLSE. Higher proportions of viable spermatozoa were seen in the presence of 0.5 and 1 μg/mL RLSE than in the control. Intracellular ROS levels decreased when the spermatozoa were preserved in BTS with 0.1–1 μg/mL RLSE. In order to examine the bacterial growth, E. coli was added to liquid semen diluted with antibiotics-free BTS in the presence or absence of RLSE. No anti-bacterial activity of RLSE against E. coli was observed during liquid semen preservation. Although there was no inhibition of E. coli growth, the addition of RLSE might help improve sperm motility and viability during boar semen preservation, suggesting it as a potential reagent for ART in mammals.
Keywords: Robus coreanus extract; diluent; spermatozoa; motility; viability; boar
Introduction
Rubus coreanus (Rosaceae) is mostly distributed in East Asian countries [1] and has been widely used in folk rem-edies for treating urogenital disorders and allergic diseases [2, 3]. As per previous reports, its components include phenolic acids, organic acids, triterpenoids, flavonoids, gallotannin, and ellagitannin [4]. In particular, phenolic and flavonoid compounds have beneficial qualities, such as free radical scavenging property [5-8], anti-inflammatory response [9], and inhibition of lipoprotein oxidation [10, 11]. Therefore, diverse biological functionalities of R. coreanus have been discussed in the context of cellular environments, including its anti-oxidant, anti-fatigue, anti-osteoporosis, anti-diabetic, and anti-bacterial properties [12-16].
As pig farms become larger and more complex, they tend to conduct breeding through artificial insemination (AI) rather than natural mating. Thus, a supply of high-quality liquid semen to pig farms is important for this industry [17]. Bacterial contamination in extended semen was associated with decreasing reproductive performances [17]. In particular, Escherichia coli (E. coli) is a harmful bacterium commonly found in pig farms and is known to reduce sperm quality during sperm storage [18]. A previous study has shown that the administration of unripe R. coreanus fruit improved the epididymal sperm number and motility in Wistar rats, suggesting its use as a nontoxic reagent for enhancing male fertility [19]. Also, R. coreanus root polyphenols showed strong anti-microbial activity against pathogenic bacteria [20].
Therefore, in the present study, boar spermatozoa were preserved in the liquid semen diluent in the presence of R. coreanus leaf and stem extract (RLSE), which was followed by examination of sperm motility, viability, intracellular reactive oxygen species (ROS) levels, and E. coli growth. The results suggest RLSE as a potential reagent to enhance fertilization competence in assisted reproductive technology for animals.
Materials and Methods
Liquid boar semen preparation
The present study was performed in accordance with the guidelines provided by the Animal Care and Use Committee (ACUC) of Chonbuk National University. Boar semen was collected from Duroc boars twice a week. Sperm concentrations were estimated using a hemocytometer, and semen was diluted with Beltsville thawing solution (BTS) [21] with or without (W/O) antibiotics (penicillin G and streptomycin), to a final concentration of 1×108 spermatozoa/mL. RLSE extract (with 99.9% methyl alcohol) was purchased from Korea Plant Extract Bank (KPEB, Ochang, Chungbuk-do, Korea). Different concentrations of RLSE (final concentration: 0–10 μg/ mL; dimethyl sulfoxide (DMSO) as a solvent control) were added to BTS. The diluted semen was stored in a storage unit at 17°C for 2 days. Unless otherwise noted, all other reagents used in this study were purchased from Sigma-Aldrich Chemical Co. LLC (St. Louis, MO, USA).
Assessment of sperm motility
Sperm motility was examined using a computer-assisted sperm analysis system (Sperm Class AnalyzerⓇ, Microptic, Barcelona, Spain). Spermatozoa were incubated for 30 min at 37.5°C, and a 1 μL aliquot of sperm sample was then placed on a pre-warmed (38°C) Leja counting slide (Leja products B.V., Nieuw-Vennep, The Netherlands). Ten fields were analyzed at 37.5°C, assessing a minimum of 500 spermatozoa per sample. The proportion of total motile spermatozoa (%), progressive motile spermatozoa (%), and hyperactive spermatozoa (%) was determined. The kinetic parameters measured for each spermatozoon included: curvilinear velocity (VCL, μm/s), straight-line velocity (VSL, μm/s), average path velocity (VAP, μm/s), percentage linearity (LIN, %), percentage straightness (STR, %), and the wobble percentage (WOB, %).
Measurement of sperm viability and intracellular ROS in spermatozoa
Incubated spermatozoa (1×108 cells/mL) were washed twice with phosphate-buffered saline containing 0.1% (w/v) polyvinyl alcohol (PBS-PVA). Sperm viability was assayed using the LIVE/DEADⓇ Sperm Viability kit (Molecular Probes, Eugene, OR, USA), which contains DNA dyes SYBR14 (100 nM) and propidium iodide (PI; 10 μM), following manufacturer’s protocol. The spermatozoa were stained, and images were acquired using a fluorescence microscope (Nikon Eclipse Ci microscope, Nikon Instruments Inc., Seoul, Korea) with camera (DS-Fi2, Nikon) and an imaging software (version 4.30, Nikon). The spermatozoa were classified and counted as viable (SYBR14) or dead (PI). The level of intracellular ROS in spermatozoa was assayed using 1 μM carboxy-DCFDA (Invitrogen, Eugene, OR, USA). The fluorescence intensity was measured using a multimode microplate reader (Spark™ 10 M, Tekan, Männedorf, Switzerland) with excitation (ex.) at 485 nm and emission (em.) at 520 nm.
Bacterial count
E. coli (ATCC 8739) were cultured in nutrient broth for 18 h at 37°C and harvested by centrifuging the cultured broth three times with PBS (pH 7.2) at 1000 × g for 10 min. E. coli stock suspension was prepared by resuspending the final pellets. Initial E. coli population ranged 1.5 × 104 CFU/mL was added by diluting the stock suspension, and the cell concentration was determined by the spreading plate method with incubation for 24 h at 37°C. During the experiments, 0.1 mL solution was withdrawn from each experimental sample and diluted 1/10 times to measure E. coli abundance. Three replicate plates were used at each dilution step.
Statistical analysis
Values are expressed as mean ± standard error of the mean (SEM). Data were analyzed using one-way analysis of variance (ANOVA) using the SAS package 9.3 (SAS Institute Inc., Cary, NC, USA) in a completely randomized design. Duncan’s multiple range test was used to compare values for individual treatments when the Fvalue was significant (p<0.05).
Results and Discussion
Improvement of sperm motility in BTS in the presence of RLSE
Boar spermatozoa were preserved with different concentrations of RLSE in BTS for 2 days. Sperm motility was determined after 30 min of incubation. Table 1 represents the sperm motility parameters across the treatment groups. The proportion of total motile spermatozoa was significantly higher in the treatment with 0.5 μg/mL RLSE than in the other groups (p<0.05; Table 1). Similarly, the percentage of progressive motile spermatozoa was significantly higher in the treatment with 0.5 μg/mL RLSE (p<0.05; Table 1). Kinetic values were not significantly different among the groups, except the VAP in 0.5 μg/mL RLSE treatment (p<0.05; Table 1). Significantly lower levels of motile spermatozoa, progressive motile spermatozoa, and VAP were seen in the DMSO treatment, a negative control (Table 1). The administration of R. coreanus fruit extract in male rodents has been shown to improve their epididymal sperm number, sperm motility, and the expression of cAMP-responsive element modulator (CREM) associated with sperm maturation [19, 22]. CREM proteins have also been detected in the connecting piece of ejaculated boar spermatozoa [23]. In this study, improved sperm motility was observed upon direct addition of RLSE to the diluent. Thus, this effect might be related to sperm function, and could prove beneficial in maintaining or improving sperm motility during liquid semen preservation.
Table 1.
Analysis of sperm motility in BTS with RLSE1
|
Parameters |
RLSE (μg/mL) in BTS |
|
0 |
0.1 |
0.5 |
1 |
2 |
5 |
10 |
DMSO |
|
Total motile spermatozoa (%) |
85.7 ± 4.1ab |
86.2 ± 3.2ab |
93.7 ± 1.4a |
88.9 ± 3.6ab |
86.4 ± 2.9ab |
84.2 ± 3.5ab |
85.5 ± 3.3ab |
82.4 ± 3.8b |
|
Progressive motile spermatozoa (%) |
70.6 ± 5.8ab |
73.0 ± 6.0ab |
85.4 ± 1.8a |
76.0 ± 6.4ab |
73.7 ± 5.1ab |
70.2 ± 4.9ab |
68.3 ± 4.5ab |
67.2 ± 6.4b |
|
Hyperactive spermatozoa (%) |
3.8 ± 3.3 |
2.0 ± 2.0 |
0.0 ± 0.0 |
3.7 ± 3.7 |
2.2 ± 2.2 |
2.5 ± 2.5 |
2.4 ± 2.4 |
5.7 ± 2.9 |
|
Kinetic values2 |
VCL (µm/s) |
72.2 ± 10.0 |
72.7 ± 2.3 |
72.6 ± 0.8 |
73.9 ± 5.7 |
72.6 ± 6.3 |
71.3 ± 5.8 |
68.1 ± 5.3 |
77.1 ± 4.6 |
|
|
VSL (µm/s) |
23.1 ± 2.0 |
32.3 ± 7.8 |
45.8 ± 3.0 |
34.3 ± 8.0 |
36.3 ± 9.2 |
35.2 ± 8.4 |
33.1 ± 8.4 |
27.0 ± 9.4 |
|
|
VAP (µm/s) |
45.9 ± 3.8b |
49.5 ± 5.4ab |
60.1 ± 0.3a |
52.3 ± 3.6ab |
52.5 ± 6.4ab |
50.8 ± 4.1ab |
48.6 ± 3.4ab |
48.5 ± 3.5ab |
|
|
LIN (%) |
34.5 ± 6.7 |
44.4 ± 10.6 |
60.7 ± 4.2 |
47.0 ± 12.4 |
49.8 ± 12.3 |
49.0 ± 12.3 |
46.7 ± 12.5 |
37.1 ± 13.8 |
|
|
STR (%) |
50.6 ± 5.7 |
61.0 ± 8.3 |
72.9 ± 4.5 |
61.8 ± 10.0 |
64.5 ± 10.1 |
64.4 ± 10.2 |
61.1 ± 10.7 |
53.3 ± 12.8 |
|
|
WOB (%) |
65.0 ± 6.0 |
67.8 ± 8.5 |
81.0 ± 0.4 |
71.4 ± 9.0 |
72.3 ± 8.3 |
71.1 ± 8.0 |
70.1 ± 8.0 |
63.8 ± 7.6 |
Download Excel Table
Sperm viability and intracellular ROS generation in boar spermatozoa preserved with RLSE
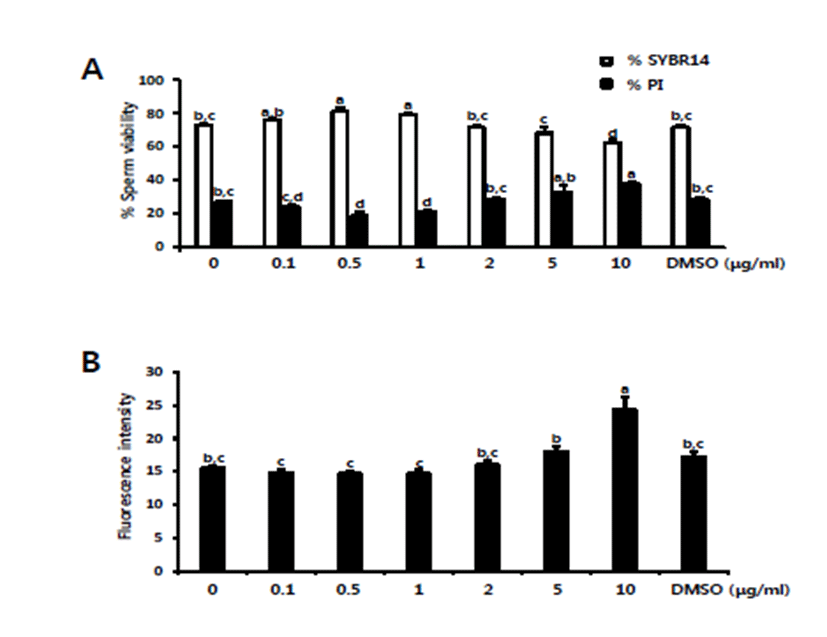
For the assessment of sperm viability, preserved spermatozoa were stained with SYBR14 and PI, followed by the counting of viable and dead spermatozoa under a fluorescence microscope. As shown in Fig. 1, significantly higher live cells were recorded in the treatments with 0.5 and 1 μg/mL RLSE (p<0.05). In contrast, the level of viable spermatozoa gradually decreased from 2 μg/mL RLSE onwards, and a significantly high percentage of dead spermatozoa were observed at 10 μg/mL RLSE (p< 0.05; Fig. 1A). Excessive ROS levels can affect sperm viability and fertilization [24, 25]. In order to measure the intracellular ROS levels, preserved spermatozoa stained with carboxy-DCFDA were subjected to fluorometric assay. The fluorescence intensity was significantly lower in the treatments with 0.1, 0.5, and 1 μg/mL RLSE than in controls and at 2–10 μg/mL concentrations (p<0.05; Fig. 1B). A recent report demonstrated that R. coreanus seed extract effectively protects DNA and protein damage by inhibiting ROS generation, owing to its polyphenol content [26]. Similarly, increased total phenolic content in Kalopanax pictus leaves resulted in better protection against DNA damage [27]. Meanwhile, total polyphenol and total flavonoid content was higher in the water extract of R. coreanus stem and leaf than the unripe water extract [28]. Therefore, RLSE could protect sperm DNA damage and regulate ROS levels during sperm preservation.
Fig. 1.
Assessment of sperm viability and intracellular ROS production after sperm preservation. Different concentrations of RLSE (0–10 μg/mL; DMSO as a control) were added to liquid boar semen, and then sperm viability (A) and intracellular ROS production (B) were examined. Experiments were repeated three times with three different boars. The values are expressed as mean ± SEM. The different superscripts (a–c) denote a significant difference between treatments at p<0.05.
Download Original Figure
Sperm motility and bacterial growth in antibioticfree BTS in the presence of RLSE
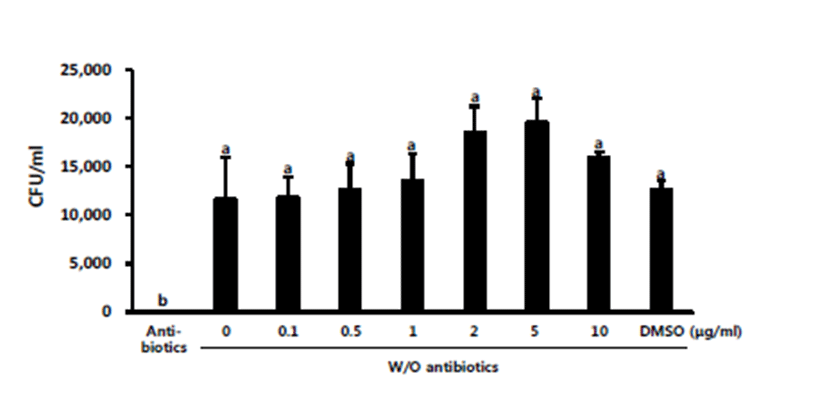
Bacteria are rarely found in the semen of healthy pigs. However, bacterial inflow may occur through the preputial diverticulum, during excretion or via dirty skin during semen collection, or due to contamination in the sampling site [29]. Bacterial contamination of semen causes sperm agglutination, acrosome damage, and decrease in sperm motility, resulting in decreased sperm viability and longevity [30, 31]. E. coli is one of the most common bacteria identified in liquid boar semen [31]. In the present study, E. coli was added to liquid semen diluted with antibiotics-free BTS. Overall, the levels of total motile spermatozoa, progressive motile spermatozoa, and sperm kinetic parameters decreased significantly in sperm samples preserved without antibiotics than in spermatozoa preserved with antibiotics (p<0.05; Table 2). Among the groups treated with different concentrations of the extract, addition of 0.5 or 1 μg/mL RLSE significantly improved the sperm motility (p<0.05; Table 2). Bacterial growth was completely inhibited in BTS containing antibiotics (Fig. 2). Although it did not differ significantly among the spermatozoa preserved in antibiotic-free BTS, the bacterial abundance was higher in groups treated with the extract than in treatments with no extract. Thus, no anti-bacterial activity of RLSE against E.coli was observed during liquid semen preservation (Fig. 2). According to Cha et al. [12], R. coreanus leaf extract with 80% methanol exhibited higher anti-bacterial activity against Bacillus cereus than unripe or ripe fruit extracts, but no anti-bacterial activity was observed against E. coli, Salmonella typhimurium, and Staphylococcus aureus, which is in agreement with our result (Fig. 2). Therefore, RLSE is expected to be effective against bacteria other than E. coli found in liquid semen, and higher concentrations of RLSE should also be tested.
Fig. 2.
The levels of growth in E. coli population in liquid boar semen in the presence of RLSE (0–10 μg/mL; DMSO as a control) after 2 days of preservation. Experiments were repeated three times with three different boars. Values are expressed as mean ± SEM. The superscripts (a & b) denote a significant difference between treatments at p<0.05.
Download Original Figure
Table 2.
Analysis of sperm motility in antibiotic-free BTS in the presence of RLSE1
|
Parameters |
Antibiotics in BTS |
RLSE (μg/mL) in ntibiotic-free BTS |
|
0 |
0.1 |
0.5 |
1 |
2 |
5 |
10 |
DMSO |
|
Total motile spermatozoa (%) |
87.0 ± 0.8a |
59.5 ± 4.6bc |
60.8 ± 8.9bcd |
70.0 ± 3.9b |
67.1 ± 2.7b |
59.3 ± 3.7bcd |
59.0 ± 2.8bcd |
43.8 ± 7.0cd |
42.3 ± 9.1d
|
|
Progressive motile spermatozoa (%) |
70.8 ± 5.2a |
23.8 ± 2.0bc |
25.5 ± 5.0bc |
31.5 ± 3.4b |
32.4 ± 8.5b |
25.6 ± 2.5bc |
20.0 ± 6.0bc |
9.0 ± 2.0c |
33.7 ± 14.9b |
|
Hyperactive spermatozoa (%) |
0.2 ± 0.2 |
0.4 ± 0.4 |
1.6 ± 0.8 |
1.8 ± 0.3 |
2.0 ± 0.8 |
2.1 ± 0.8 |
1.1 ± 0.6 |
0.6 ± 0.3 |
1.2 ± 0.7 |
|
Kinetic values2 |
VCL (µm/s) |
69.4 ± 5.6a |
42.1 ± 1.2bc |
43.5 ± 3.8b |
47.9 ± 3.3b |
50.5 ± 8.9b |
46.4 ± 1.0b |
37.6 ± 7.9bc |
26.9 ± 2.6c |
39.1 ± 1.7bc |
|
|
VSL (µm/s) |
20.3 ± 2.3a |
9.8 ± 0.9bc |
10.0 ± 0.9bc |
10.6 ± 0.2b |
11.4 ± 3.0b |
10.6 ± 1.0b |
8.5 ± 2.1bc |
5.3 ± 0.8c |
7.9 ± 0.4bc |
|
|
VAP (µm/s) |
34.7 ± 3.4a |
19.4 ± 1.1b |
19.9 ± 1.8b |
21.5 ± 1.1b |
23.1 ± 5.1b |
21.1 ± 1.0b |
17.0 ± 4.3bc |
10.6 ± 1.5c |
16.9 ± 0.8bc |
|
|
LIN (%) |
27.8 ± 2.1a |
20.5 ± 3.4abc
|
22.1 ± 1.2b |
20.9 ± 4.4abc
|
17.7 ± 3.3bc |
17.4 ± 1.8bc |
18.0 ± 2.0bc |
12.3 ± 1.9c |
16.6 ± 2.3bc |
|
|
STR (%) |
54.5 ± 2.7a |
45.5 ± 4.3b |
47.6 ± 0.9b |
44.2 ± 4.3b |
42.1 ± 4.2b |
44.7 ± 2.5b |
45.0 ± 1.2b |
41.8 ± 4.0b |
41.5 ± 3.5b |
|
|
WOB (%) |
48.2 ± 1.7a |
40.6 ± 2.9b |
41.8 ± 0.8b |
41.8 ± 3.5b |
39.0 ± 2.8b |
36.8 ± 1.9bc |
37.6 ± 2.6b |
29.4 ± 3.3bc |
36.6 ± 2.2bc |
Download Excel Table
In conclusion, the addition of RLSE in the diluent improved sperm motility and viability, and modulated the ROS level during liquid semen preservation. However, there was no inhibition of E. coli growth, and the antibacterial activity of RLSE warrants further investigation. Overall, the results suggest that RLSE is a potentially beneficial reagent for assisted reproductive technology in mammals.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2013R1A6A3A04063769).
References
Choi HS, Kim MK, Park HS, Kim YS, Shin DH. Alcoholic fermentation of Bokbunja (Rubus coreanus Miq.) wine. Korean J Food Sci Technol. 2006; 38:543-547.

Shin TY, Kim SH, Lee ES, Eom DO, Kim HM. Action of Rubus coreanus extract on systemic and local anaphylaxis. Phytother Res. 2002; 16:508-513.

Ku CS, Mun SP. Characterization of seed oils from fresh Bokbunja (Rubus coreanus Miq.) and wine processing waste. Bioresour Technol. 2008; 99:2852-2856.

Yoon I, Wee JH, Moon JH, Ahn TH, Park KH. Isolation and identification of quercetin with antioxidative activity from the fruits of Rubus coreanum Miquel. Korean J Food Sci Technol. 2003; 35:499-502.

Sato M, Ramarathnam N, Suzuki Y, Ohkubo T, Takeuchi M, Ochi H. Superoxide radical scavenging activities of wines, and antioxidative properties of fractions recovered from merlot wine pomace. Food Factors for Cancer Prevention. 1977Springer
Frankel EN, Kanner J, German JB, Park SE, Kinsella JE. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993; 20:454-457.

Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001; 73:285-290.

Yilmaz Y, Toledo RT. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J Food Composit Analy. 2006; 19:41-48.

Moroney MA, Alcaraz MJ, Forder RA, Carey F, Hoult JR. Selectivity of neutrophil 5-lipoxygenase and cyclooxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J Pharm Pharmacol. 1988; 40:787-792.

Serafini M, Ghiselli A, Ferro-Luzzi A. Red wine, tea, and antioxidants. Lancet. 1994; 27:626.

Teissedre PL, Frankel EN, Waterhouse AL, Peleg H, German JB. Inhibition of in vitro human LDL oxidation by phenolic antioxidants from grapes and wines. J Sci Food Agri. 1996; 70:55-61.

Cha H, Park MS, Park KM. Physiological activities of Rubus coreanus Miquel. Korean J Food Sci Technol. 2001; 33:409-415.

Bae JY, Lim SS, Choi JS, Kang YH. Protective actions of Rubus coreanus ethanol extract on collagenous extracellular matrix in ultraviolet-B irradiation induced human dermal fibroblasts. Nutri Res Prac. 2007; 1:279-284.

Yoon HJ, Kim CS, Lee KY, Yang SY. Antioxidant activity of Rubus coreanus fruit extract in comparison to green tea extract. Chonnam Med J. 2010; 46:148-155.

Choi C, Lee H, Lim H, Park S, Lee J, Do S. Effect of Rubus coreanus extracts on diabetic osteoporosis by simultaneous regulation of osteoblasts and osteoclasts. Menopause. 2012; 19:1043-1051.

Om AS, Song YN, Noh G, Kim H, Choe J. Nutrition Composition and Single, 14-Day and 13-Week Repeated Oral Dose Toxicity Studies of the Leaves and Stems of Rubus coreanus Miquel. Molecules. 2016; 21:65.

Althouse GC, Pierdon MS, Lu KG. Thermotemporal dynamics of contaminant bacteria and antimicrobials in extended porcine semen. Theriogenology. 2008; 70:1317-1323.

Pinart E, Domènech E, Bussalleu E, Yeste M, Bonet S. A comparative study of the effects of Escherichia coli and Clostridium perfringens upon boar semen preserved in liquid storage. Anim Reprod Sci. 2017; 177:65-78.

Oh MS, Yang WM, Chang MS, Park W, Kim DR, Lee HK, Kim WN, Park SK. Effects of Rubus coreanus on sperm parameters and cAMP-responsive element modulator (CREM) expression in rat testes. J Ethnopharmacol. 2007; 114:463-467.

Kim SK, Kim H, Kim SA, Park HK, Kim W. Antiinflammatory and anti-superbacterial activity of polyphenols isolated from black raspberry. Korean J Physiol Pharmacol. 2013; 17:73-79.

Pursel V, Johnson LA. Frozen boar spermatozoa methods of thawing pellets. J Anim Sci. 1976; 42:927-931.

Vogt PH. Molecular genetic of human male infertility from genes to new therapeutic perspectives. Curr Pharm Des. 2004; 10:471-500.

Noda T, Shidara O, Harayama H. Detection of the activator cAMP responsive element modulator (CREM) isoform ortholog proteins in porcine spermatids and sperm. Theriogenology. 2012; 77:1360-1368.

Silva P, Gadella B. Detection of damage in mammalian sperm cells. Theriogenology. 2006; 65:958-978.

Yi YJ, Lee IK, Lee SM, Yun BS. An antioxidant davallialactone from Phellinus baumii enhances sperm penetration on in vitro fertilization of pigs. Mycobiology. 2016; 44:54-57.

Choi MH, Shim MS, Kim GH. Protective effect of black raspberry seed containing anthocyanins against oxidative damage to DNA, protein, and lipid. J Food Sci Technol. 2016; 53:1214-1221.

Hu W, Heo SI, Wang MH. Antioxidant and anti-inflammatory activity of kalopanax pictus leaf. J Korean Soc Appl Biol Chem. 2009; 52:360-366.

Lee MJ, Lee SJ, Choi HR, Lee JH, Kwon JW, Chae KS, Jeong JT, Lee TB. Improvement of cholesterol and blood pressure in fruit, leaf and stem extracts from black raspberry in vitro. Korean J Medicinal Crop Sci. 2014; 22:177-187.

Kuster CE, Althouse GC. The impact of bacteriosper- Effect of RLSE on boar spermatozoa mia on boar sperm storage and reproductive performance. Theriogenology. 2016; 85:21-26.

Althouse GC, Kuster CE, Clark SG, Weisiger RM. Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology. 2000; 53:1167-1176.

Althouse GC, Lu KG. Bacteriospermia in extended porcine semen. Theriogenology. 2005; 63:573-584.