Introduction
Panniculitis is a skin disease characterized by inflammation of the subcutaneous fat tissues [1]. Sterile nodular panniculitis (SNP) appears as a single or multiple deepseated nodules of varying sizes [2]. The skin lesions often become fistulated and drain an oily substance [2-4]. Additionally, systemic signs including a fever, inappetence, and lethargy wax and wane along with the skin changes [4].
In cases of SNP, histopathology reveals pyogranulomatous or granulomatous inflammation originating in the subcutis rather than the dermis [5]. SNP may progress to a more diffuse pattern with necrosis and fistulous tracts extending into the dermis and epidermis [6]. A diagnosis of SNP can be made after ruling out infectious and other noninfectious causes [1].
Dapsone, also known as diaminodiphenyl sulfone, has a combination of antimicrobial, antiprotozoal, and anti-inflammatory effects [7]. It has been used in the treatment of various immune-mediated dermatoses, such as pemphigus complex [8, 9], vasculitis [10, 11], and neutrophilic dermatitis [12, 13]. SNP is treated with immunomodulatory drugs including prednisolone, azathioprine, and cyclosporine [2], while the usefulness of dapsone is yet unknown. This report describes the clinical application of dapsone in two cases of SNP.
Case Report
A 12-year-old, neutered male, Maltese dog was presented to the Veterinary Medical Center (VMC) of Chungbuk National University for generalized ulcerative dermatitis. Two years ago, the skin lesions began as papules in the perianal area. Intermittently, the dog was treated with steroids over an 18-month period, but the clinical symptoms recurred repeatedly.
Two months prior to presentation, skin lesions developed on the head, which then progressed to crusts and multifocal ulcers. These lesions spread to the trunk, dorsum, limbs, gluteal region, and abdomen within 2 days. The dog also developed systemic signs including pyrexia, lethargy, and anorexia. Because there was no clinical response to antibacterial and antifungal drugs, the dog was treated with leflunomide at the local hospital. With this treatment, the clinical symptoms improved but was not resolved completely.
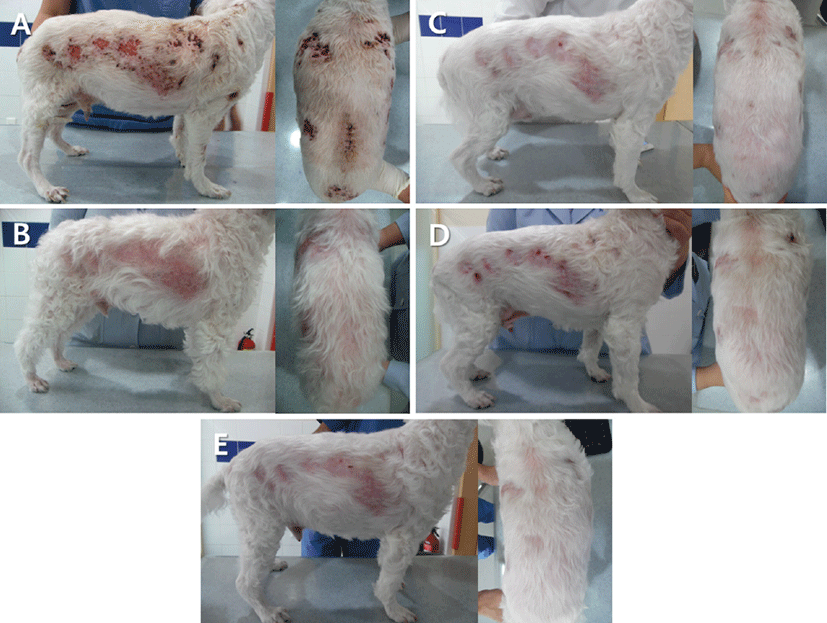
On presentation to the VMC, dermatological examination revealed multifocal alopecia, erythema, ulcers, and crusts on the trunk, dorsum, limbs, gluteal region, and abdomen (Fig. 1A). Depression and anorexia were also noted.
A hematological analysis revealed moderate anemia (packed cell volume: 21.5%, reference range: 37.3- 61.7%) and severe neutrophilic leukocytosis (white blood cell count: 40,770/μL, reference range: 5,050- 16,760/μL; neutrophils: 35,918/μL, reference range: 2,950-11,640/μL). Hypoalbuminemia (albumin: 1.5 g/ dL, reference range: 2.6-3.3 g/dL) and increased alkaline phosphatase (ALP) level (1,375 IU/L, reference range: 29-97 IU/L) were noted on the serum chemistry panel.
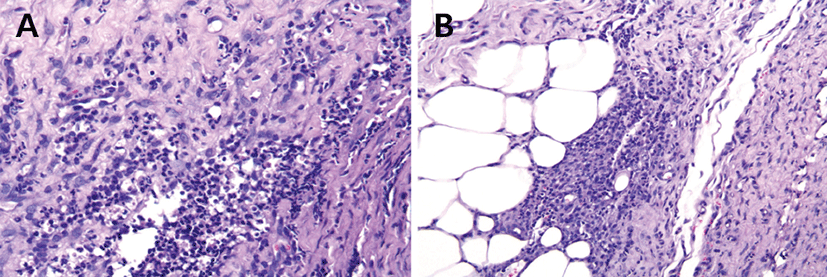
Impression smears obtained from the ulcerative skin lesions demonstrated numerous degenerate neutrophils, macrophages, and a few acantholytic cells. No etiological agents were observed on the clear taping, trichogram, skin scraping, and cultures for bacteria and fungi. Initially, amoxicillin-clavulanic acid (25 mg/kg, PO, twice daily; Clavamox, Zoetis, Korea), itraconazole (5 mg/ kg, PO, once daily; Sponazol, Hankook Nelson Pharm, Korea), and ivermectin (300 μg/kg, PO, once daily; Ivomec, Merial, Brazil) were prescribed for 7 days. Because skin lesions were inflamed, multiple punch biopsies were obtained from the trunk. Histopathology revealed pyogranulomatous nodular to diffuse dermatitis, hyperplasia, and hypertrophy of the dermis, and panniculus adiposus (Fig. 2). The inflammatory cells were composed principally of macrophages and neutrophils infiltrating the dermis (Fig. 2A) and panniculus (Fig. 2B). Additionally, tissue sections were stained with periodic acid schiff (PAS), hematoxylin and eosin, and FITE’s (acid-fast) to rule out fungal and mycobacterial infection. However, no microorganisms were detected. Therefore, the dog was definitively diagnosed with SNP based on the clinical and histopathological findings as well as the negative results from the special stainings.
Immunosuppressive treatment with prednisolone (2 mg/ kg, PO, twice daily; Solondo, Yuhan, Korea) and cyclosporine (5 mg/kg, PO, once daily; Cypol-N, Chongkundang, Korea) was initiated. The ulcerative skin lesions began to gradually improve 10 days after the initiation of therapy. Because the skin lesions were nearly resolved after 113 days of therapy (Fig. 1B), the immunosuppressive drugs were discontinued after an additional 21 days of therapy. However, the erythematous nodules and crusts recurred on the trunk, dorsum, and neck 28 days after ceasing treatment (Fig. 1C). To prevent hepatotoxicity caused by continued steroid use, the dog was treated with dapsone (1 mg/kg, PO, twice daily; Dapsone, Taiguk, Korea) for 6 days. Therapy with prednisolone and cyclosporine was then restarted due to a poor response to dapsone (Fig. 1D). There was no recurrence of the skin lesions at the 10 week follow-up appointment (Fig. 1E).
A 9-year-old, intact male, Shih-Tzu dog was referred to the VMC with a history of recurrent skin lesions, such as papules and nodules, over a period of 5 years. A nodule in the right gluteal region ruptured 3 days prior to presentation.
On presentation, multiple skin lesions were palpated over the entire body. The skin lesions were comprised of erythematous, painful, and ruptured nodules on the dorsum, gluteal region, and front feet. Results of the complete blood cell count were within the reference intervals. The serum chemistry panel demonstrated mildly increased ALP level (324 IU/L, reference range: 29-97 IU/L), and the remainder of the values were within the reference intervals.
Impression smears obtained from the ruptured lesions revealed numerous neutrophils, macrophages, red blood cells, and a few eosinophils. Fine needle aspiration of a nodule in the gluteal region showed inflammatory cell infiltrates including degenerate neutrophils and macrophages. Radiographs did not reveal bone involvement or the presence of a foreign body. No etiological agents were observed on the clear taping, trichogram, skin scraping, and cultures for bacteria and fungi.
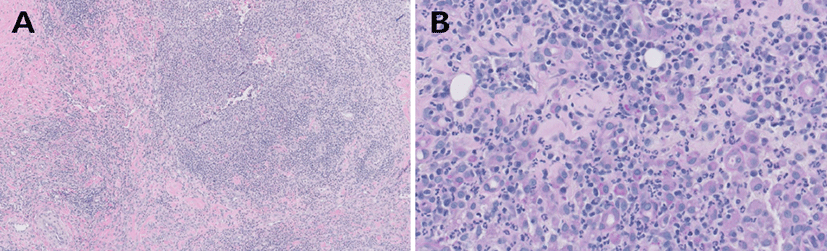
Skin biopsies were obtained from the nodular lesions on the dorsum and gluteal region. The histopathologic results demonstrated diffuse pyogranulomatous dermatitis (Fig. 3). Cellular infiltrates were observed in the subcutis. The predominant inflammatory cells were neutrophils, histiocytes, and fibrocytes, while microorganisms were not observed. Additionally, tests with special stainings including PAS, hematoxylin and eosin, and FITE did not reveal any microorganisms. Therefore, the dog was definitively diagnosed with SNP based on clinical and histopathological findings.
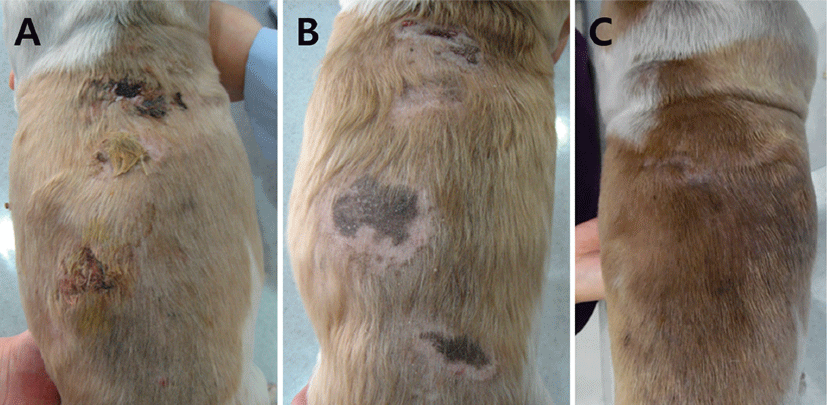
Initially, an immunomodulatory dose of prednisolone (1 mg/kg, PO, twice daily; Solondo, Yuhan, Korea) was administered. The skin lesions gradually improved, and the prednisolone dose was tapered to 0.25 mg/kg, twice daily after 56 days of therapy. The prednisolone dose then had to be increased (to 0.5-0.75 mg/kg, PO, twice daily) for an additional 50 days due to recurrence of the lesions. When the dog was examined 106 days after initiation of therapy, the skin lesions remained (Fig. 4A). Because of the low efficacy of prednisolone in this case, the prescription was changed to triamcinolone (0.4 mg/kg, PO, once daily; Tracinon, Chodang, Korea) plus azathioprine (2 mg/kg, PO, once daily; Immuthera, Celltrion, Korea). Five days later, severely elevated levels of ALP (29,100 IU/L, reference range: 29-97 IU/L) and aspartate aminotransferase (142 IU/L, reference range: 21-102 IU/L) were noted. Treatment was changed to dapsone (1 mg/ kg, PO, three times a day; Dapsone, Taiguk, Korea) due to presumed steroid-induced hepatotoxicity. The skin lesions improved with the introduction of dapsone (Fig. 4B) so it was continued for another 44 days. There was no evidence of recurrence of SNP 5 months after cessation of treatment with dapsone (Fig. 4C).
Discussion
Because the etiology and pathology of SNP remains unknown, definitive diagnosis of the disease is difficult. Therefore, a diagnosis of SNP should be made only after ruling out all possible infectious or other noninfectious causes by utilizing a multidisciplinary diagnostic approach [1, 14, 15]. Generally, a definitive diagnosis of SNP is based on history, clinical appearance, histopathologic features, the absence of microbiological infection, and the absence of other identifiable causes such as trauma, foreign body, insect bite, and drug eruption. Systemic symptoms including pyrexia, lethargy, and anorexia must also be present [2, 7, 15, 16]. In the present two cases, the possibility of microbiological infection was ruled out based on basic dermatological examination results and non-detection of microorganisms in cultures and special staining tests. Both dogs had systemic signs, such as pyrexia, lethargy, and anorexia. A histopathological analysis demonstrated diffuse pyogranulomatous dermatitis. Therefore, the two dogs were definitively diagnosed with SNP based on clinical and histopathological findings.
In the management of SNP with multiple lesions, treatment with immunosuppressive drugs is commonly used [2, 14]. SNP should be managed with immunosuppressive therapy until achieving remission of clinical signs [6]. Antibiotics should be used to reduce inflammation and drainage when secondary bacterial infections are present [6]. There are reports that describe the synergistic effects of tetracycline and niacinamide used in combination [17]. Though their mechanism of action is unknown, it has been demonstrated that this combination is able to inhibit lymphoblast formation and chemotaxis of neutrophils and eosinophils [18].
In the present two cases, dapsone was prescribed in lieu of steroids due to the presence of presumed steroid- induced hepatotoxicity. Generally, dapsone has the dual function of antimicrobial/antiprotozoal and antiinflammatory activities. Because the latter capabilities resemble those of non-steroidal anti-inflammatory drugs, dapsone could also be useful in treating chronic inflammatory diseases. Additionally, because of its ability to inhibit leukocytes, it may be useful in the management of immune-mediated dermatoses, such as pemphigus complex, vasculitis, and neutrophilic dermatitis [7, 19].
Although both of the cases in this report were diagnosed with SNP, their clinical response to dapsone was opposite. In the first case, combination therapy with prednisolone and cyclosporine was useful in attenuating the ulcerative lesions, while dapsone alone was not effective in managing the clinical signs. In contrast, in the second case, the therapeutic response to common immunomodulatory drugs, such as prednisolone, triamcinolone, and azathioprine was inadequate. Interestingly, dapsone alone was effective in controlling the clinical signs without causing undue side effects. Generally, dapsone is used for the treatment of immune-mediated dermatoses of mild to moderate severity [2]. Because the skin lesions in the second case were relatively mild in comparison to those in the first case, dapsone may have been more effective in managing the clinical signs observed in the second case.
In conclusion, this report describes the therapeutic effects of dapsone in two dogs with SNP. For the treatment of SNP, current guidelines recommend combination therapy with oral glucocorticoids (prednisolone) and calcineurin inhibitors (cyclosporine) rather than monotherapy [20]. If patients require long–term therapy to remain in remission, drugs alternative to glucocorticoids are advisable. Because the therapeutic responses and side effects vary with each case, it is important that appropriate drugs are selected with a case-specific approach. Although the usefulness of dapsone in the management of canine SNP is yet unknown, it may be considered in cases of mild to moderate SNP when the use of steroids is not recommended due to its low efficacy or side effects.