Original Article
Protective effect of the aerial parts of Silybum marianum against amyloid β protein (25-35)-induced neuronal death in cultured neurons
Hae Min Lee, Ji Yeon Jang, Yeon Hee Seong*

College of Veterinary Medicine and Veterinary Medical Center, Chungbuk National University, Cheongju 28644, Korea
*Corresponding author: Yeon Hee Seong, College of Veterinary Medicine, Chungbuk National University, Cheongju 28644, Korea +82-43-261-2968, +82-43-267-2595, E-mail:
vepharm@cbnu.ac.kr
© Research Institute of Veterinary Medicine, Chungbuk National University. All rights reserved.
Received: Dec 5, 2016; Revised: Dec 20, 2016; Accepted: Dec 20, 2016
Abstract
Alzheimer’s disease (AD), a progressive neurodegenerative disorder that deprives the patient of memory, is associated mainly with extracellular senile plaque induced by the accumulation of amyloid β protein (Aβ). Silybum marianum (Asteraceae; SM) is a medicinal plant that has long been used in traditional medicine as a hepatoprotective remedy owing to its antioxidant and anti-inflammatory activities. The present study examined the methanol extract of the aerial parts of SM for neuroprotection against Aβ (25-35)-induced neuronal death in cultured rat cortical neurons to investigate a possible therapeutic role of SM in AD. The primary cortical neuron cultures were prepared using embryonic day 15 to 16 SD rat fetuses. Cultured cortical neurons exposed to 10 μM Aβ (25-35) for 36 h underwent neuronal cell death. At 10 and 50 μg/mL, SM prevented Aβ (25-35)-induced neuronal cell death and apoptosis in cultured cortical neurons. Furthermore, SM inhibited the Aβ (25-35)-induced decrease in anti-apoptotic protein, Bcl-2, and the increase in the proapoptotic proteins, Bax and active caspase-3. Cultured cortical neurons exposed to 1 mM N-methyl-D-aspartate (NMDA) for 14 h induced neuronal cell death. SM (10 and 50 μg/mL) prevented NMDA-induced neuronal cell death. These results suggest that SM inhibited Aβ (25-35)-induced neuronal apoptotic death via inhibition of NMDA receptor activation and that SM has a possible therapeutic role in preventing the progression of neurodegeneration in AD.
Keywords: Silybum marianum; amyloid β protein; neuroprotection; cultured neurons; N-methyl-D-aspartate
Introduction
Alzheimer’s disease (AD), the most common form of the senile dementia, is a progressive neurodegenerative disorder that deprives the patient of memory, eventually leading to death. AD is associated mainly with extracellular senile plaque induced by the accumulation of amyloid β protein (Aβ), a 39-43 amino acid fragment derived from the amyloid precursor protein [1]. Many studies have indicated that Aβ neurotoxicity might involve oxidative stress, excitotoxicity induced by glutamate release, and resulting apoptotic neuronal death [2-4]. Therefore, the blockade of these pathways is of major interest in the prevention and treatment of AD. In addition, the deposition of Aβ in the pathogenesis of AD is invariably associated with the inflammatory responses [5]. Antioxidants, such as α-tocopherol, offer protection against Aβ-induced cytotoxicity and the development of learning and memory deficits, and anti-inflammatory agents, such as indomethacin, slow the progression of AD [6, 7].
Silybum marianum (Asteraceae; SM) is a medicinal plant found throughout the world; its therapeutic history dates back to 2000 years ago as a hepatoprotective medication to treat jaundice [8]. Numerous experimental and clinical studies have shown that SM with its antioxidant activity is a unique hepatoprotective agent [9]. Silymarin, the main component of SM, is highly hepatoprotective and is used for the treatment of numerous liver disorders [10, 11]. Although its mechanisms of action are not completely understood, it appears that it has antioxidant and antiinflammatory activities, cell permeability regulating and membrane stabilizing properties, and liver regenerative effects [11-13]. Most studies of SM were about liver disorders; however, it has beneficial properties on a wide variety of other disorders, such as renal protection, cardiovascular protection, cancer, and Alzheimer prevention [14]. SM protects cultured hippocampal neurons against oxidative stress-induced cell death, which suggests that SM contains beneficial chemicals on the nervous system [15]. In support of this idea, the flavonoid silibinin, the major active constituent of silymarin, prevented the dopaminergic neuronal loss in a mouse Parkinson’s disease model and inhibited acetylcholinesterase (AChE) and Aβ peptide aggregation for the treatment of AD [16, 17]. Therefore, it was hypothesized that SM might protect neurons against neurodegeneration in AD. Most studies focused on the seed extract of SM (silymarin); the aerial parts, including leaves, stems, and flowers, of the plant are usually discarded. This study examined the protective effects of the methanol extract of the aerial parts of SM on Aβ (25-35)-induced neuronal cell death in primary cultured rat cortical neurons.
Materials and Methods
Plant materials and extraction and reagents
Methanol extract of the aerial part of SM was generously provided by Herbal Crop Research Division, National Institute of Horticultural and Herbal Science, Rural Development Administration, in Eumseong, Chungbuk, Korea. SM was farmed and harvested in this organization in 2005. SM was extracted using 100% methanol at 50℃ and filtered. The filtrate was concentrated under reduced pressure using a rotary evaporator, which was then stored at room temperature. Aβ (25-35) was purchased from Bachem (Bubendorf, Switzerland). 3-[4,5-Dimethylthiazol- 2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) and N-methyl-D-aspartate (NMDA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Hoechst 33342 dye was supplied by Molecular Probes Inc. (Eugene, OR, USA). Fetal bovine serum was obtained from JRS Biosciences (Lenexa, KS, USA). Proprep buffer was acquired from iNtRONBio. Inc. (Gyunggi-Do, Korea). Rabbit polyclonal antibodies against Bcl-2, Bax, active caspase-3, β-actin, and horseradish peroxidase-conjugated anti-rabbit secondary antibody were purchased from Millipore Inc. (Bedford, MA, USA). Horseradish peroxidase-conjugated anti-goat secondary antibody was obtained from Assay Designs (Ann Arbor, MI, USA).
Experimental animals
Pregnant Sprague-Dawley (SD) rats for the primary neuronal culture were supplied by Daehan BioLink Co., Ltd. (Chungbuk, Korea) and housed in an environmentally controlled room at 22 ± 2℃, with a relative humidity of 55 ± 5%, a 12-h light/dark cycle, and food and water ad libitum. The procedures involving the experimental animals complied with the animal care guide lines of the U. S. National Institutes of Health and the Animal Ethics Committee of Chungbuk National University.
Induction of neurotoxicity and analysis of neuronal viability in primary cultures of rat cerebral cortical neurons
The primary cortical neuron cultures were prepared using embryonic day 15 to 16 SD rat fetuses, as described elsewhere [18]. The neurotoxicity experiments were performed on the neurons after 4-5 days in culture. An Aβ (25-35) stock solution of 2 mM was prepared in sterile distilled water, stored at -20℃, and incubated for more than 2 days at 37℃ to aggregate prior to use. Cultured neurons were treated with 10 μM Aβ (25-35) in serumfree DMEM at 37℃ for 36 h to produce neurotoxicity. To measure the NMDA-induced neuronal death, the neurons were treated with 1 mM NMDA in a HEPES-buffered solution containing HEPES (8.6 mM), NaCl (154 mM), KCl (5.6 mM), and CaCl2 (2.3 mM) at pH 7.4 for 14 h at 37℃. SM was dissolved in dimethyl-sulfoxide (DMSO) at a concentration of 100 mg/mL and diluted further in experimental buffers. The final concentration of DMSO was less than 0.1%, which did not affect the cell viability. For each experiment, SM was applied 20 min prior to treatment with 10 μM Aβ (25–35) or 1 mM NMDA. SM was also present in the medium during Aβ (25–35) or NMDA incubation. At the end of the incubation period, the viability of the neuronal cells was monitored using a colorimetric MTT assay and Hoechst 33342 staining, as described elsewhere [18].
Western blotting
Cultured neurons treated with 10 μM Aβ (25-35) with or without SM for 36 h on dishes were lysed with the Pro- Prep buffer. The total proteins were extracted, and Western blot analysis of Bcl-2, Bax, and active caspase-3 expression was performed as described in a previous study [19] (Kim et al., 2011). The amount of protein was measured using a Bicinchoninic acid assay [20]. Protein expression was detected using an enhanced chemiluminescence detection reagent (ELPIS Biotech, Co., Daejoen, Korea). The images were quantified using image analysis software (Image J 1.45S, a freely available application in the public domain for image analysis and process, developed and maintained by Wayne Rasband at the Research Services Branch, National Institutes of Health).
Statistical analysis
The data is expressed as the mean ± standard error of the mean (S.E.M.), and the statistical significance was assessed by one-way analysis of variance (ANOVA) followed by a Tukey’s tests. P values <0.05 were considered significant.
Results
Protective effect of SM against Aβ (25-35)-induced neuronal cell death
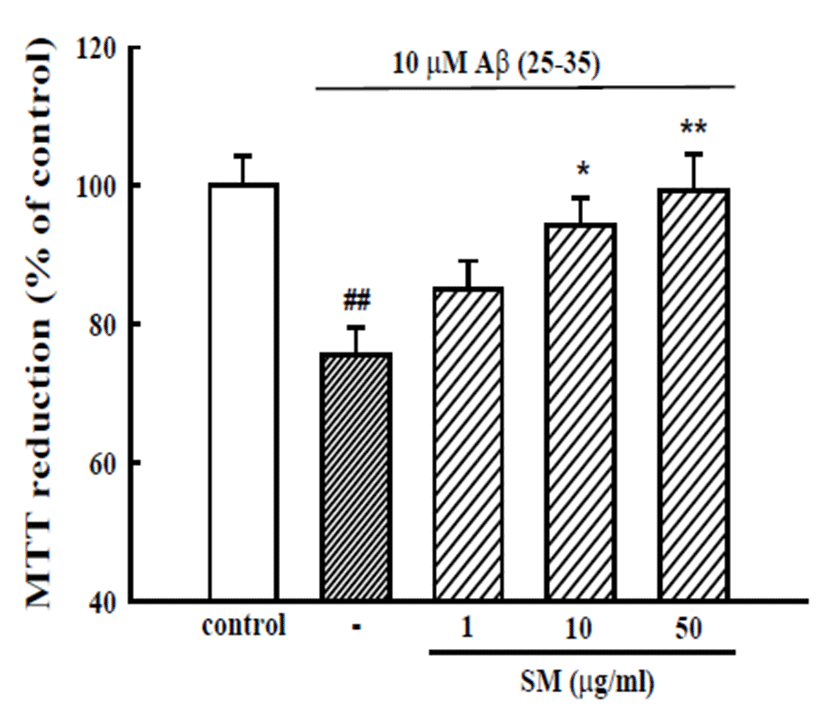
Cultured cortical neurons exposed to 10 μM Aβ (25- 35) for 36 h showed 75.6 ± 3.9% of the absorbance of untreated controls in a MTT assay, indicating that Aβ (25-35) caused neuronal cell death. The pretreatment of cortical neurons with 10 and 50 μg/mL SM reduced the neuronal death induced by Aβ (25-35) (absorbance, 94.3 ± 4.0% and 99.4 ± 5.2% of control, respectively; Fig. 1).
Fig. 1.
Inhibitory effect of SM on Aβ (25-35)-induced neuronal cell death in cultured cortical neurons. Neuronal cell death was measured using a MTT assay. The MTT absorbance from the untreated cells was normalized to 100% as a control. The results are expressed as the mean ± SEM of the data obtained from 5 independent experiments performed in three or four wells. ##p<0.01 vs. control; *p<0.05, **p < 0.01 vs. 10 μM Aβ (25-35).
Download Original Figure
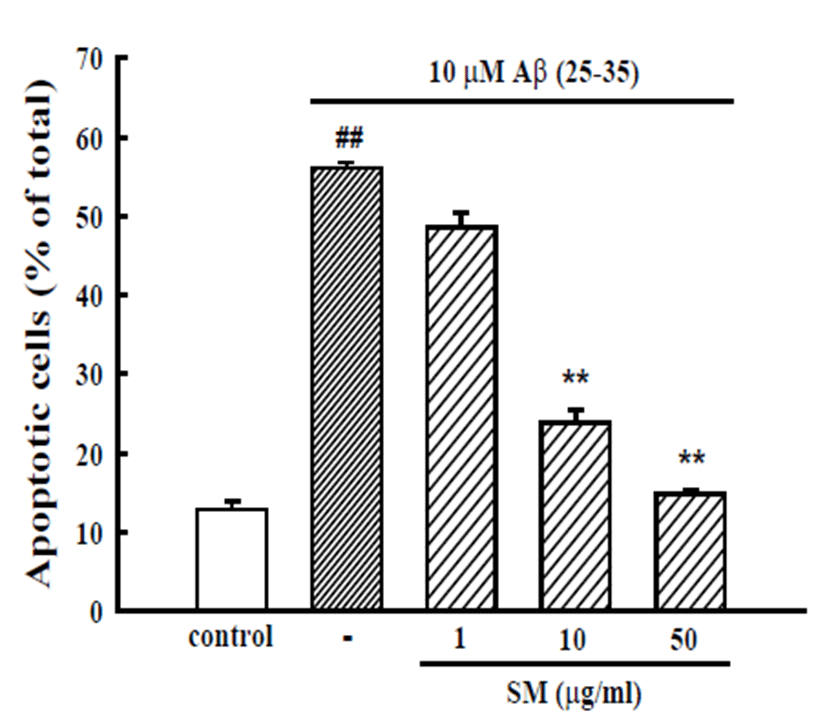
An additional experiment was performed with Hoechst 33342 staining to detect the condensed or fragmented DNA, which is indicative of Aβ (25-35)-induced neuronal apoptotic death. The treatment of neurons with 10 μM Aβ (25-35) induced the apoptosis of 56.1 ± 0.7% of the total population of cultured cortical neurons compared to 12.8 ± 1.0% apoptotic neurons in the control cultures. The addition of SM (10 and 50 μg/mL) decreased the Aβ (25-35)-induced apoptotic cell death significantly, showing 23.9 ± 2.7 and 14.8 ± 0.8%, respectively (Fig. 2).
Fig. 2.
Inhibitory effects of SM on Aβ (25-35)-induced apoptosis of cultured cortical neurons. Apoptotic cells measured by Hoechst 33342 staining were counted in 3 fields per well. The values represent the apoptotic cells as a percentage of the total number of cells expressed as the mean ± SEM of data obtained from 3 independent experiments. ##p < 0.01 vs. control; **p < 0.01 vs. 10 μM Aβ (25-35).
Download Original Figure
Inhibitory effect of SM on Aβ-induced change of apoptosis-associated proteins
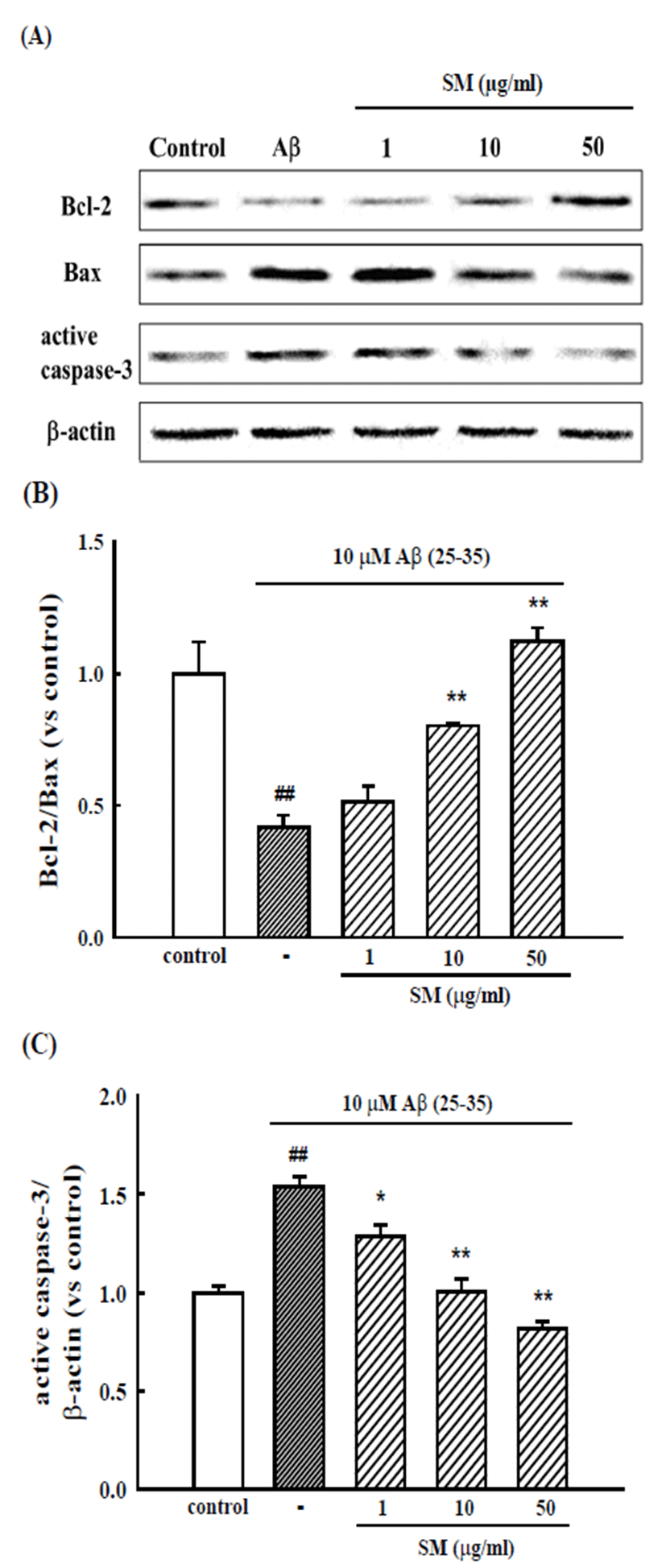
Cultured cortical neurons exposed to Aβ (25-35) exhibited increased expression of the pro-apoptotic proteins, Bax and active caspase-3, and decreased expression of anti-apoptotic protein, Bcl-2. The pretreatment of cortical neurons with SM (10 and 50 μg/ml) inhibited the changes in these pro-apoptotic and anti-apoptotic proteins (Fig. 3).
Fig. 3.
Inhibitory effects of SM on Aβ (25-35)-induced expression of apoptosis-associated proteins. (A) Representative Western blots of the pro-apoptotic and anti-apoptotic proteins from the cultured cortical neurons. Bar graphs showing the relative ratio of Bcl-2/Bax (B), and active caspase-3/β-actin (C) expression versus control. The results are expressed as the mean ± SEM of the data obtained from 4 independent experiments. ##p<0.01 vs. control; *p<0.05, **p < 0.01 vs. 10 μM Aβ (25- 35).
Download Original Figure
Protective effect of SM against NMDA-induced neuronal cell death
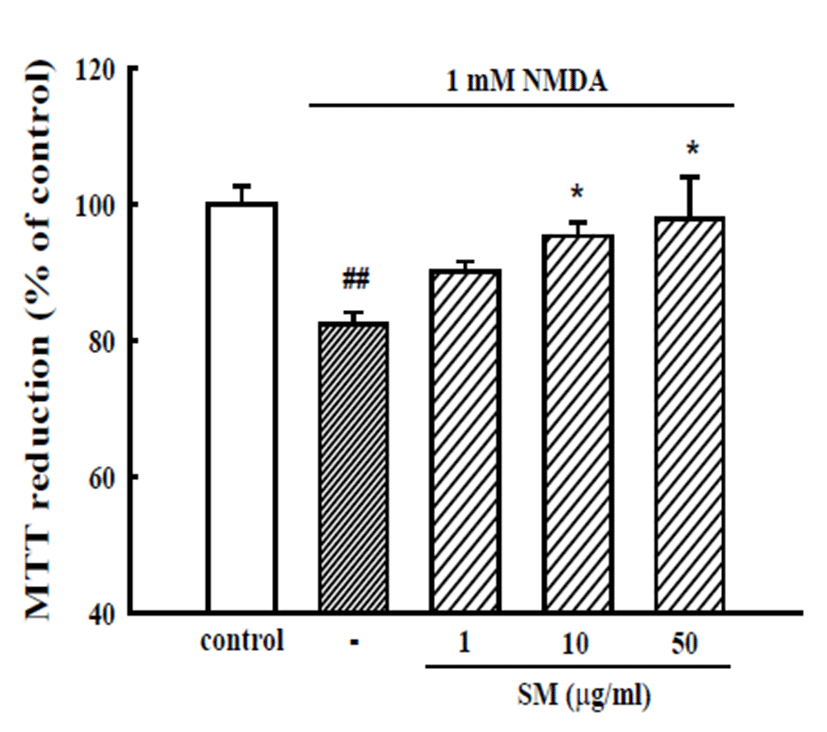
Cultured cortical neurons exposed to 1 mM NMDA for 14 h showed 82.4 ± 1.7% absorbance of that of the untreated controls in a MTT assay, indicating that NMDA caused neuronal cell death. The pretreatment of cortical neurons with 10 and 50 μg/mL SM reduced the NMDAinduced neuronal death (absorbance, 95.3 ± 2.0% and 97.8 ± 6.2% of control, respectively) (Fig. 4).
Fig. 4.
Inhibitory effects of SM on NMDA-induced neuronal cell death in cultured cortical neurons. Neuronal cell death was measured by a MTT assay. The absorbance of the untreated neurons is regarded as 100%. The results are expressed as the mean ± S.E.M. values of the data obtained from three independent experiments performed in three or four wells. ##p<0.01 vs. control; *p<0.05 vs. 1 mM NMDA.
Download Original Figure
Discussion
Aβ (25-35), which is the core toxic fragment of full length Aβ (1-40), forms a β-sheet structure and induces neuronal cell death, neuritic atrophy, synaptic loss, and memory impairments [21, 22]. Aβ (25-35) was reported to cause neuronal cell death, as shown in the present study. The mechanisms underlying Aβ-neurotoxicity are complex but may involve the NMDA receptor, a glutamate receptor subtype, activation induced by glutamate release followed by the sustained increase of intracellular Ca2+, and the generation of reactive oxygen species (ROS), finally triggering neuronal death [2, 3, 23]. This assumption was reinforced by the observations that Aβ (25-35)-induced neuronal death was blocked by MK- 801, an NMDA receptor antagonist; verapamil, a L-type Ca2+ channel blocker; and L-NG-nitroarginine methyl ester (L-NAME), a nitric oxide synthase inhibitor, in cultured neurons [24, 25]. The present study provides evidence that Aβ (25-35)-induced injury to rat cortical neurons can be prevented by a methanol extract of the aerial parts of SM. SM could reduce NMDA-induced neuronal cell death in the current study. These results suggest that SM might prevent Aβ (25-35)-induced Ca2+ entry through the NMDA-receptor-coupled channels and ROS generation to inhibit neuronal death. SM may stabilize the membranes in a manner that blocks Ca2+ influx via the NMDA receptor-coupled voltage dependent Ca2+ channels and inhibits ROS generation. Supporting this hypothesis, studies have shown that silymarin, the main component of SM, has cell permeability regulating properties as well as membrane stabilizing properties and anti-oxidant activity [12, 26]. Furthermore, antioxidants, such as including silymarin, catechin, and curcumin, could inhibit glutamate-induced neurotoxicity in PC12 cells [27].
Many in vitro neuronal experiments have shown that Aβ is accompanied by multiple events culminating in apoptosis [28, 29]. Bax, which is a member of the Bcl- 2 family that resides mainly in the cytosol of healthy cells, translocates to the mitochondria after exposure to Aβ and increases the release of cytochrome c from the mitochondria [30]. Cytosolic cytochrome c forms a functional apoptosome that activates caspase-9 and caspase-3. Numerous proteins are cleaved by activated caspase-3, which initiates the biochemical cascades that lead to cell death [31, 32]. SM decreased significantly the Aβ (25-35)-induced apoptotic cell death, as measured by Hoechst 33342 staining. Furthermore, SM inhibited the Aβ (25-35)-induced decrease in the anti-apoptotic protein, Bcl-2, and increase in pro-apoptotic proteins, Bax and active caspase-3. Therefore, these results suggest that the neuroprotective effect of SM might be due to the prevention of apoptotic neuronal death by suppressing NMDA-induced Ca2+ entry and ROS generation due to the anti-oxidant components of this plant.
Silymarin, a polyphenolic flavonoid, is isolated from SM and is a combination of some bioflavonoids found in fruits, seeds, and leaves of this plant, including silibinin, silybin, isosilybin, silydianin, and silychristin [11, 33]. Silibinin, a major active constituent of silymarin, prevented Aβ-induced memory impairment in mice through its antioxidative and anti-inflammatory activity [34, 35]. To the best of the authors’, this is the first report to demonstrate the neuroprotective effects of the aerial parts of SM against Aβ (25-35)-induced neuronal death in primary cultured neurons. In conclusion, the present study suggests that SM containing a variety of free radical scavenging agents can inhibit Aβ (25-35)-induced neuronal apoptotic death by inhibiting NMDA receptor activation.
Acknowledgements
We thank Dr. Seung Eun Lee, Herbal Crop Research Division, National Institute of Horticultural and Herbal Science, Rural Development Administration, in Eumseong, Chungbuk, Korea, for providing with methanol extract of Silybum marianum (pj00962903).
References
Selkoe D.J. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001; 81:741-766.

Butterfield D.A., Drake J., Pocernich C., Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol. Med. 2001; 7:548-554.

Mattson M.P., Chan S.L. Neuronal and glial calcium signaling in Alzheimer's disease. Cell Calcium. 2003; 34:385-397.

Parks J.K., Smith T.S., Trimmer P.A., Bennett J.P., Parker W.D.. Neurotoxic Abeta peptides increase oxidative stress in vivo through NMDA-receptor and nitric-oxide-synthase mechanisms, and inhibit complex IV activity and induce a mitochondrial permeability transition in vitro. J. Neurochem. 2001; 76:1050-1056.

Gitter B.D., Cox L.M., Rydel R.E., May P.C. Amyloid beta peptide potentiates cytokine secretion by interleukin- 1 beta-activated human astrocytoma cells. Proc. Natl. Acad. Sci. USA. 1995; 92:10738-10741.

Gasparini L., Ongini E., Wenk G. Non-steroidal antiinflammatory drugs (NSAIDs) in Alzheimer's disease: old and new mechanisms of action. J. Neurochem. 2004; 91:521-536.

Sano M., Ernesto C., Thomas R.G., Klauber M.R., Schafer K., Grundman M., Woodbury P., Growdon J., Cotman C.W., Pfeiffer E., Schneider L.S., Thal L.J. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N. Engl. J. Med. 1997; 336:1216-1222.

Sewell R.D., Rafieian-Kopaei M. The history and ups and downs of herbal medicine usage. J HerbMed Pharmacol. 2014; 3:1-3.

Negi A., Kumar J., Luqman S., Shanker K., Gupta M., Khanuja S. Recent advances in plant hepatoprotectives: A chemical and bilolgical profile of some important leads. Med. Res. Rev. 2008; 28:746-772.

Jayaraj R., Deb U., Bhaskar A.A., Prasad G.B., Rao P.V. Hepatoprotective efficacy of certain flavonoids against microcystin induced toxicity im mice. Environ. Toxicol. 2007; 22:472-479.

Karimi G., Vahabzadeh M., Lari P., Rashedinia M., Moshiri M. Silymarin, a promising pharmacological agent for treatment of diseases. Iran. J. Basic Med. Sci. 2011; 14:308-317.

Muthumani M., Prabu S.M. Silibinin potentially attenuates arsenic-induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Cardiovasc. Toxicol. 2014; 14:83-97.

Nazemian F., Karimi G., Moatamedi M., Charkazi S., Shamsara J., Mohammadpour A.H. Effect of silymarin administration on TNF-alpha serum concentration in peritoneal dialysis patients. Phytother. Res. 2010; 24:1654-1657.

Bahmani M., Shirzad H., Rafieian S., Rafieian-Kopaei M. Silybum marianum: Beyond Hepatoprotection. J. Evid. Based Complementary Altern. Med. 2015; 20:292-301.

Kittur S., Wilasrusmee S., Pedersen W.A., Mattson M.P., Straube-West K., Wilasrusmee C., Lubelt B., Kittur D.S. Neurotrophic and neuroprotective effects of milk thistle (Silybum marianum) on neurons in culture. J. Mol. Neurosci. 2002; 18:265-269.

Duan S., Guan X., Lin R., Liu X., Yan Y., Lin R., Zhang T., Chen X., Huang J., Sun X., Li Q., Fang S., Xu J., Yao Z., Gu H. Silibinin inhibits acetylcholinesterase activity and amyloid beta peptide aggregation: a dual-target drug for the treatment of Alzheimer's disease. Neurobiol. Aging. 2015; 36:1792-1807.

Lee Y., Park H.R., Chun H.J., Lee J. Silibinin prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease via mitochondrial stabilization. J. Neurosci. Res. 2015; 93:755-765.

Ban J.Y., Jeon S.Y., Bae K., Song K.S., Seong Y.H. Catechin and epicatechin from Smilacis chinae rhizome protect cultured rat cortical neurons against amyloid beta protein (25-35)-induced neurotoxicity through inhibition of cytosolic calcium elevation. Life Sci. 2006; 79:2251-2259.

Kim J.Y., Jeong H.Y., Lee H.K., Yoo J.K., Bae K., Seong Y.H. Protective effect of Ilex latifolia, a major component of "kudingcha", against transient focal ischemia-induced neuronal damage in rats. J. Ethnopharmacol. 2011; 133:558-564.

Brenner A.J., Harris E.D. A quantitative test for copper using bicinchoninic acid. Anal. Biochem. 1995; 226:80-84.

Pike C.J., Walencewicz-Wasserman A.J., Kosmoski J., Cribbs D.H., Glabe C.G., Cotman C.W. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25-35 region to aggregation and neurotoxicity. J. Neurochem. 1995; 64:253-265.

Tohda C., Matsumoto N., Zou K., Meselhy M.R., Komatsu K. A beta (25-35)-induced memory impairment, axonal atrophy, and synaptic loss are ameliorated by M1, A metabolite of protopanaxadiol-type saponins. Neuropsychopharmacology. 2004; 29:860-868.

Wang R., Reddy P.H. Role of Glutamate and NMDA Receptors in Alzheimer's Disease. J. Alzheimers Dis. 2016.

Cho S.O., Ban J.Y., Kim J.Y., Jeong H.Y., Lee I.S., Song K.S., Bae K., Seong Y.H. Aralia cordata protects against amyloid beta protein (25-35)-induced neurotoxicity in cultured neurons and has antidementia activities in mice. J. Pharmacol. Sci. 2009; 111:22-32.

Jeong H.Y., Kim J.Y., Lee H.K. Ha do T, Song KS, Bae K, Seong YH. Leaf and stem of Vitis amurensis and its active components protect against amyloid beta protein (25-35)-induced neurotoxicity. Arch. Pharm. Res. 2010; 33:1655-1664.

Jayaraj R., Deb U., Bhaskar A.S., Prasad G.B., Rao P.V. Hepatoprotective efficacy of certain flavonoids against microcystin induced toxicity in mice. Environ. Toxicol. 2007; 22:472-479.

Mazzio E., Huber J., Darling S., Harris N., Soliman K.F. Effect of antioxidants on L-glutamate and N-methyl- 4-phenylpyridinium ion induced-neurotoxicity in PC12 cells. Neurotoxicology. 2001; 22:283-288.

Ekinci F.J., Linsley M.D., Shea T.B. Beta-amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Brain Res. Mol. Brain Res. 2000; 76:389-395.

Yan X.Z., Qiao J.T., Dou Y., Qiao Z.D. Beta-amyloid peptide fragment 31-35 induces apoptosis in cultured cortical neurons. Neuroscience. 1999; 92:177-184.

Zong W.X., Lindsten T., Ross A.J., MacGregor G.R., Thompson C.B. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001; 15:1481-1486.

Estus S., Tucker H.M., van Rooyen C., Wright S., Brigham E.F., Wogulis M., Rydel R.E. Aggregated amyloid-beta protein induces cortical neuronal apoptosis and concomitant "apoptotic" pattern of gene induction. J. Neurosci. 1997; 17:7736-7745.

Nicholson D.W., Thornberry N.A. Caspases: killer proteases. Trends Biochem. Sci. 1997; 22:299-306.

Pepping J. Milk thistle: Silybum marianum. Am. J. Health Syst. Pharm. 1999; 56:1195-1197.

Lu P., Mamiya T., Lu L.L., Mouri A., Niwa M., Hiramatsu M., Zou L.B., Nagai T., Ikejima T., Nabeshima T. Silibinin attenuates amyloid beta(25-35) peptide-induced memory impairments: implication of inducible nitricoxide synthase and tumor necrosis factor-alpha in mice. J. Pharmacol. Exp. Ther. 2009; 331:319-326.

Lu P., Mamiya T., Lu L.L., Mouri A., Zou L., Nagai T., Hiramatsu M., Ikejima T., Nabeshima T. Silibinin prevents amyloid beta peptide-induced memory impairment and oxidative stress in mice. Br. J. Pharmacol. 2009; 157:1270-1277.