Introduction
Rotaviruses are considered as the most important etiological agents of acute viral gastroenteritis in young animals and children throughout the world [1, 2]. The viruses belong to the family Reoviridae, and their genome consists of 11 segments of dsRNA, encoding six structural proteins (VP1 to VP4, VP6 and VP7) and six non-structural proteins (NSP1 to NSP6) [3]. Their particles are icosahedral and composed of the triple concentric layers, outer capsid, inner capsid and core capsid, which surround the dsRNA segments [4]. Viral proteins on its external layer are composed of VP4 and VP7, and the middle layer contains the major inner capsid protein (VP6). The major outer protein VP6 in double-layered (single-shelled) particles, constitutes 50-60% of the total mass of group A virion [5]. It is highly antigenic and immunogenic, and thus frequently used to detect rotavirus antigen and antibody in immunological methods. As it also possesses group epitope specificity, rotaviruses were classified into seven groups (A to G) [4, 6-8].
Group C rotavirus (GpC-RV) was first recognized as viral etiologic agent causing diarrhea in piglets in 1980, and the first identification of GpC-RV in human was reported in 1982 and then, the viral agents have been responsible for much of public health burden so far [9-12]. Antigenicity and dsRNA migration pattern by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) between human and porcine GpC-RVs were similar, but those of group A, B, D and E RVs were distinct although they were morphologically identical [13]. Cell-culture adaptation and propagation of GpC-RV, a prototype porcine Cowden strain, has been successful, but accomplished to date with few non GpA-RV strains [14]. Antibodies against bovine GpC-RV have been detected in serum of cattle worldwide, and two strains (Shintoku and porcine-like WD534tc) have been reported so far [11, 15, 16].
In this study, VP6 proteins were expressed using a bacroulovirus expression system to develop diagnostic tests for detection of GpC-RV. Furthermore, we produced and characterized monoclonal antibodies(mAbs) against VP6 protein of bovine GpC-RV expressed on baculovirus expression system.
Materials and Methods
Bovine fecal samples confirmed as GpC-RV positive by RT-PCR were obtained from Dr. Cho, College of Veterinary Medicine, Chonnam National University in Korea. Viral RNA was extracted from fecal samples using QIAamp® viral RNA mini kit (Qiagen, Germany) and directly used for RT-PCR. Based on the reference sequences retrieved from Genbank, a pair of primers including XhoI and HindIII restriction enzyme sites (underline) was designed to amplify bovine GpC-RV VP6 gene. The sense primer was VP6-F (5’ - GATCTCGAG TCATGGATGTGCTGTTCTCCAT-3’), while the antisense primer was VP6-R (5’ - ATCAAGCTTCATCACCATTCTCTTCACGGA-3’). PCR amplification was carried out on a thermal cycler (TaKaRa, Japan) with reaction mixture: 5μL of cDNA template obtained from RT reaction, 10pmol/μL of each primer, 4μL of 10X PCR buffer, 4μL of dNTP, 24.5μL of sterilized distilled water and Ex Tap DNA polymerase (TaKaRa) in a final volume of 40μL. Thermal conditions were as follows: initial denaturation at 95°C for 5min, 35cycles with denaturation at 95°C for 1min, annealing at 60°C for 1min and extension at 72°C for 1min 30sec, and a final extension of 5min at 72°C. The PCR products were examined with 1 Kb DNA ladder (Invitrogen Life Technologies, USA) on 1% agarose gel containing ethidium bromide.
For cloning of bovine GpC-RV VP6 gene , the amplified PCR products were digested with restriction enzymes XhoI and HindIII (Takara), purified using the ExpinTM Gel SV kit (GeneAll, Korea), and then inserted into corresponding regions of pBlueBac4.5/V5-His-TOPO® vector (Invitrogen) using T4 DNA ligase. The ligation mixture was transformed into competent cells (HIT DH5-α, RBC) and grown on a LB agar plate with ampicillin (50 μg/mL). Successful insertion of VP6 genomic DNA was verified by PCR amplification and nucleotide sequencing using a BigDye Terminator Cycle Sequencing Ready Reaction kit and PRISM 3730XL DNA analyzer (Applied Biosystems, USA).
For expression of bovine GpC-RV VP6 gene, recombinant baculoviruses expressing bovine GpC-RV VP6 gene were produced by co-transfecting the transfer vector using Bac-N-blue™ transfection kit (Invitrogen) in Sf9 cells according to the manufacturer’s protocol. Each recombinant baculovirus was selected and plaque-purified using X-gal. To confirm the presence of the inserted GpC-RV VP6 gene in recombinant baculoviruses, PCR was performed using GpC-RV VP6 and baculovirus specific primers. Indirect fluorescence antibody (IFA) and Western blotting test were applied to confirm the expression of GpC-RV VP6 gene in the recombinant baculoviruses. For IFA, commercial anti-V5 specific antibody (Bethyl Laboratories, USA) and FITC-conjugated goat anti-rabbit IgG + M (Jackson ImmunoResearch Laboratories, USA) were used as a primary and secondary antibody, respectively. In addition, Western blotting was performed by the standard method [17] using anti-V5 specific antibody and alkaline phosphatase-conjugated goat anti-rabbit IgG + M (Jackson ImmunoResearch Laboratories).
Bovine GpC-RV VP6-specific mAbs were produced using expressed bovine GpC-RV VP6. For production of GpC-RV VP6-specific mAbs, BALB/c mice were immunized with purified VP6. VP6 was purified from bovine GpC-RV VP6 recombinant baculovirus, rGpC/VP6 [136-1] - infected Sf9 cell lysates using QIA express® Ni-NTA Fast (Qiagen) according to manufacturer’s instructions. Purified VP6 was emulsified with an equal volume of Freund’s complete adjuvant (Sigma, USA) and then inoculated on each footpad of an 8-week-old female BALB/c mouse (Samtako Biokorea, Korea). The inoculation was repeated two times every 3 days. After 3 days following final inoculation, popliteal lymph nodes and spleen were removed aseptically from the immunized mouse, and then lymphocytes and splenocytes were fused with SP2/0 myeloma cells according to the modified procedures of a previous study [18]. The GpC-RV VP6 positive hybridomas were screened by IFA test and cloned at least twice by limiting dilution methods. The isotype of each mAb was determined by mouse monoclonal antibody isotyping reagents (Sino Biological, China) according to the manufacturer protocol.
Cross-reactivity of the bovine GpC-RV VP6-specific mAbs with the closely-related recombinant baculoviruses and different serotypes of reference human and animal GpA-RVs was assessed by IFA test described previously [19]. Recombinant baculoviruses are previously described [19] as follows : porcine GpC-RV (Cowden strain) VP6 recombinant virus (rGpC/VP6 [Cowden]), porcine GpC-RV (Korean isolate) VP6 recombinant virus (rGpC/VP6 [Kor]), human GpA-RV (Wa strain) VP6 recombinant virus (rGpA/VP6 [Wa]). Different serotypes of reference human and animal GpA-RVs are as follows : human rotaviruses Wa (serotype 1), DS-1 (serotype 2), M (serotype 3), ST3 (serotype 4) and F45 (serotype 9), simian rotavirus SA-11 (serotype 3), porcine rotaviruses Gottfried (serotype 4) and OSU (serotype 5), bovine ro taviruses NCDV (serotype 6), ATCC (serotype 6), I-801 (serotype 8) and B223 (serotype 10), avian rotavirus AEQ (serotype 7). Porcine rotavirus Cowden strain was used as a reference GpC-RVs.
Results
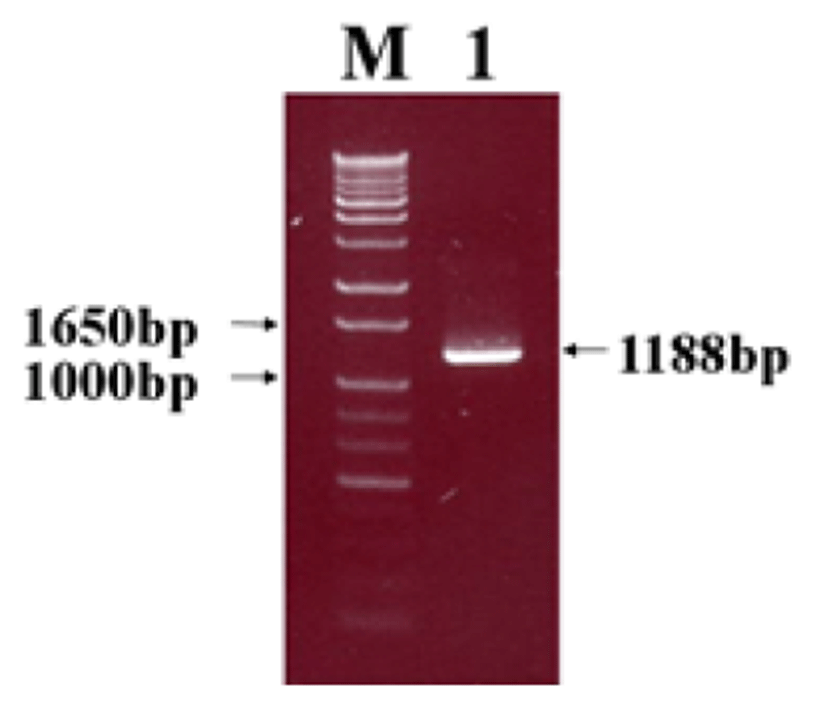
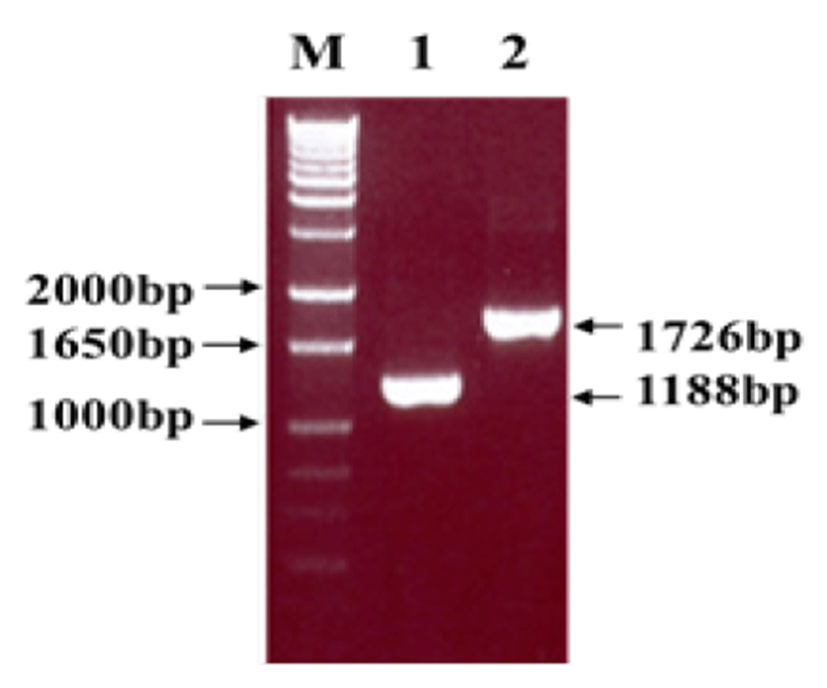
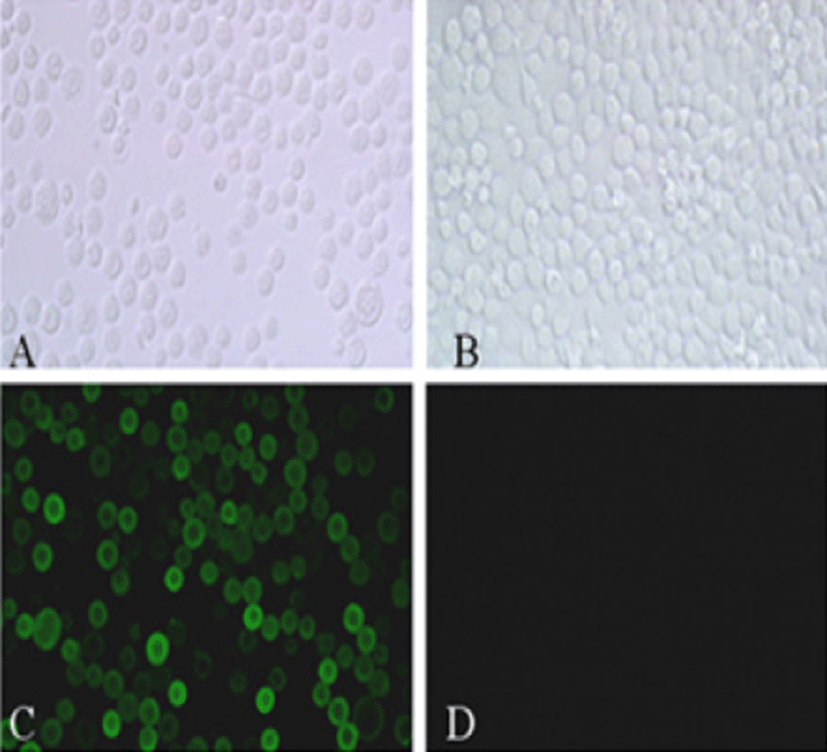
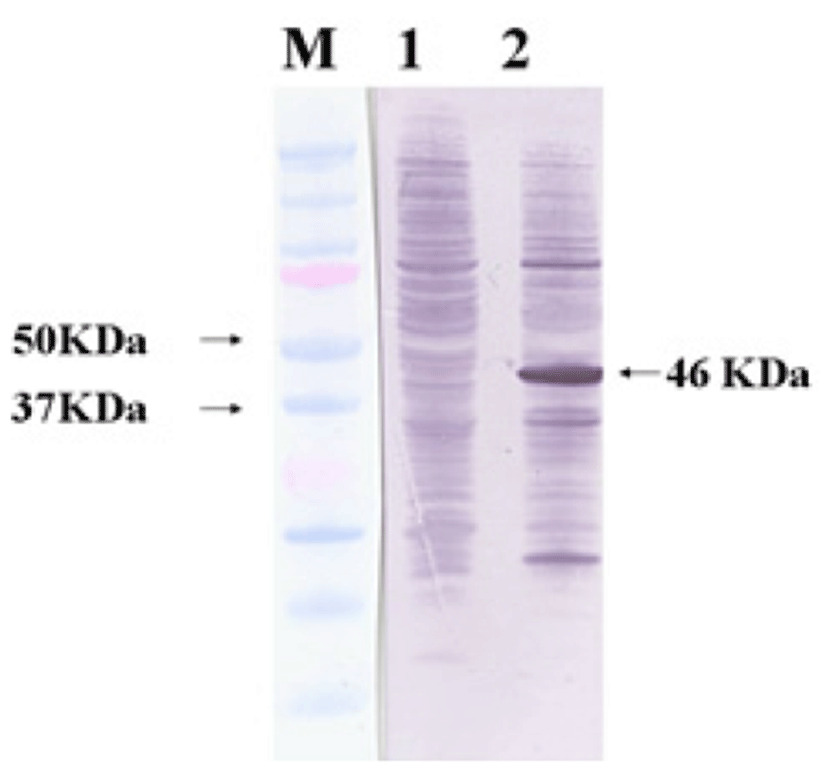
Viral RNA was extracted from bovine GpC-RV fecal sample, and RT-PCR was performed with bovine GpC-RV VP6 gene-specific primer pairs, which resulted in the amplicon with expected size of 1,188bp for the VP6 gene (Fig. 1). Cloned genes were expressed by baculovirus expression system, which was verified by IFA, PCR and Western blot assays for successful expression. To confirm GpC-RV VP6 gene in the recombinant baculovirus, rGpC/VP6 [136-1], PCR was performed using GpC-RV VP6 and the baculovirus-specific primer set. In this experiment, amplified DNA of expected sizes (1,188 bp and 1,726 bp, respectively) was identified (Fig. 2). Specific cytopathic effects such as swelling or shrinking and detachment from the surface of the cells were observed on Sf9 cells infected with recombinant baculovirus, rGpC/VP6 [136-1] but not on mock-infected Sf9 cells at 48hr post infection (Fig. 3). As expected, Western blotting analysis showed the presence of a 46 kDa protein band in Sf9 cell lysates infected with rGpC/VP6 [136-1] (Fig. 4).
Eleven hybridomas that secret bovine GpC-RV VP6-specific mAbs were produced from cell fusion. The isotype of 6 mAbs (1G2, 2B12, 4H2, 5F6, 6A3 and 6C9) was IgG1, whereas the others were identified as IgG2b (Table 1). Antigenic specificity of each mAbs was confirmed by IFA using GpC-RV VP6 recombinant viruses (rGpC/VP6 [Cowden] and rGpC/VP6 [Kor]) and GpA-RV VP6 recombinant virus (rGpA/VP6 [Wa]). Seven mAbs (2D2, 2E3, 4H2, 5A6, 5F6, 6A3 and 6C9) reacted with all porcine and bovine GpC-RV VP6 recombinant viruses, whereas 4 mAbs (1D2, 2A4, 2B11 and 2B12) did not react with porcine GpC-RV VP6 recombinant virus, rGpC/VP6 [Cowden]. None of the mAbs were reactive with GpA-RV VP6 recombinant virus (Table 1).
The reactivity patterns of mAbs with different serotypes of reference human and animal GpA-RV were examined by IFA. As expected, all mAbs did not react with GpA-RV strains used. However, 7 mAbs except 4 mAbs (1D2, 2A4, 2B11 and 2B12) reacted with intact porcine GpC-RV Cowden strain (Table 2).
Discussion
Group A rotaviruses (GpA-RV) have been known to be major causative agents in gastroenteritis of animals including infants, and their characteristics were investigated at molecular levels to develop effective vaccines [1]. On the other hand, group C rotavirus (GpC-RV) infections have been gradually increasing worldwide, and studies on the GpC-RV have been hampered by the lack of an in vitro culture system [13, 20]. For this reason, the generation of GpC-RV-specific mAbs has been particularly important for studies of the virus structure, antigenic diversity, epitope mapping, viral diagnosis and vaccine development. This study is aimed to develop the diagnosis of GpC-RV based on detection of antigen/antibody by constructing and expressing GpC-RV VP6 gene and producing the mAbs against expressed protein.
Protective genes of some viruses such as Noroviruses and Papillomaviruses, which did not grow in vitro culture system, were expressed on prokaryotic and/or eukaryotic expression systems [21, 22]. Baculovirus is widely used for viral gene expression, and numerous recombinant proteins have been produced using this system [23, 24]. IFA, PCR and Western blot assays were used to confirm the expression of VP6 gene by the recombinant baculovirus. For Western blot assay, a comparable size of VP6 protein was identified with a 46-kDa band, although molecular weight of intact rotavirus VP6 is 42-kDa. Ojeh et al [25] reported that Cowden strain rotavirus VP6-specific mAb reacted with 41-kDa of protein. Also, Tosser et al [26] reported that VP6 gene products expressed using baculovirus expression system were identified at 43-kDa size by Western blot assay. The difference between molecular weight of the VP6 in whole intact virus and the baculovirus-expressed VP6 might be attributed to the fact that pBlueBac 4.5/V5-His-TOPO® vector used for cloning in this study includes 6 histidine and V5 tag at the carboxyl terminus.
The investigation on cross-reactivity between the mAbs and related recombinant baculoviruses and/or different serotypes of reference human and animal GpA rotaviruses showed that all mAbs reacted with bovine group C rotavirus VP6 recombinant virus, rGpC/VP6 [136-1], but none of the mAbs reacted with reference GpA-RV strains used. Therefore, these results indicated that the mAbs obtained in this study are specific for GpC-RV VP6 protein. Tsunemitsu et al [27] produced some mAbs against GpC-RV VP6 which cross-reacted with GpA-RV VP6. However, we could not generate cross-reacting mAbs with GpA-RV. However, it is very interesting that some of the mAbs (1G2, 2A4, 2B11 and 2B12) recognized the epitope of the porcine GpC-RV (Korean isolate) recombinant baculovirus, rGpC/VP6 [Kor] but not that of the porcine GpC-RV (Cowden strain) recombinant baculovirus, rGpC/VP6 [Cowden]. As expected, these mAbs did not react with intact rotavirus Cowden strain. It has been known that Vp6 of group A rotavirus has a common antigenic site and is divided into two subgroups (Subgroup I/II) depending on the antigenic differences, and mAbs specifically reacted with these subgroups were produced and characterized [28, 29]. If a GpC-RV VP6 had epitopes which determine the subgroup specificity like those of the GpA-RV VP6, porcine and bovine GpC-RV Korean isolate could belong to the same subgroup, and four mAbs would be useful to differentiate the different subgroups of GpC-RV. Further studies will be needed to define the subgroup specificity in a GpC-RV VP6 using more GpC-RV isolates.
In conclusion, we obtained recombinant protein of GpC-RV VP6 gene and at least two different groups of mAbs that specifically bind to different epitopes. VP6 recombinant protein and mAbs produced in this study are expected to be useful for the development of immunodiagnostic test such as rapid diagnostic kits, IFA and ELISA and for antigenic mapping of GpC-RV VP6 gene.