Introduction
Centella asiatica, commonly known as Gotu Kola and belonging to the family Mackinlayaceae, is a species native to Asia, listed as a treatment for dementia in the ancient Indian Ayurvedic medical text, Caraka Susmita [1, 2]. Several therapeutic properties have been to attributed to this perennial herb in the Indian system of medicine for the treatment of several disorders, including wound healing, insanity, eczema, ulcers, leprosy, and for asthma [3, 4] In addition, numerous scientific reports have documented its anxiolytic, anti-inflammatory, anti-viral, nerve stimulatory, diuretic, adaptogenic, and anti-stress properties [5, 6]. Centella asiatica is also used to re-vitalize the brain and nervous system, attention span, and concentration [7]. Preliminary studies on the effects of Centella asiatica on the central nervous system suggest that extracts of this herb are well tolerated and may have pro-cognitive effects in humans and rodents. Centella asiatica improves memory retention in rodents [8, 9] and increases performance and behavior in mentally retarded children [10]. Additional uses of Centella asiatica include promotion of a deep state of relaxation and mental calmness during meditation practices as well as alleviation of depression and anxiety when combined with other herbs [11].
Several studies have provided evidence that the mechanisms of action of Centella asiatica are relevant to neurodegenerative disease therapeutics [12]. The neuroprotective effect of Centella asiatica has been demonstrated following exposure of cultured neurons to glutamate [13]. In addition, Centella asiatica reverses the effect of pentylenetetrazole (PTZ), a GABAA antagonist, and protects against PTZ-induced convulsions and ATPase inhibition [14, 15]. Moreover, Centella asiatica treatment was shown to reduce protein carbonyl production in brains of aged rats [16]. A recent study also demonstrated that Centella asiatica extract has a protective effect on amyloid pathology in PSAPP mice with Alzheimer's disease (AD) expressing both amyloid precursor protein and presenilin 1 mutations [10]. These data suggest that Centella asiatica may reduce neurodegenerative disease-related neuropathology. Previous studies have also shown Centella asiatica to ameliorate cognitive impairment and neurotoxicity in animal models, although its effect on neural stem cell differentiation has not been studied.
In this study, we demonstrated the effect of Centella asiatica on differentiation of cultured mouse neural stem cells and PC12 neuroectodermal neuronal cells, as well as its neuroprotective effect against oxidative stress.
Materials and Methods
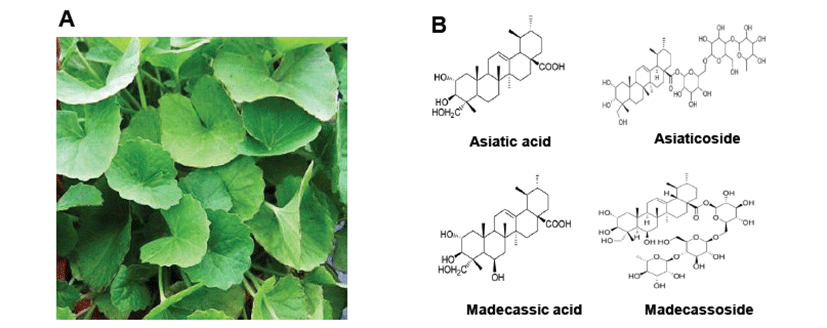
Centella asiatica extract was obtained from Dong Kook Pharmaceutical Co., Ltd. (Seoul, Korea). Centella asiatica leaf (Fig. 1A) was extracted with 60~80% ethanol at reflux extraction tank. Centella asiatica extract was concentrated under reduced pressure to distill ethanol. Solids were recovered by filtration, concentrated, pulverized, and dried. Main bioactive compounds of Centella asiatica are Asiatic acid, Asiaticoside, Madecassic acid and Madecassoside as shown in Fig. 1B.
Mice were housed and bred under specific pathogen-free conditions at the Laboratory Animal Research Center of Chungbuk National University, Korea (CBNUA-436-12-02). Mice were maintained in a room with a constant temperature of 22 ± 1°C, relative humidity of 55 ± 10%, and 12-hr light/dark cycle and fed standard rodent chow and purified tap water ad libitum. Neural stem cells were isolated from embryonic day 18.5 (E18.5) forebrain germinal zones from ICR mice (Daehan biolink, Korea). Bulk cultures were established, and the medium included DMEM/F12, 10% FBS, and 1% penicillin/streptomycin. After 24 hr, the medium was changed with Neurobasal medium containing 1% glutamate, N2 supplement, B27 supplement, 100 U/mL of penicillin, and 100 μg/mL of streptomycin.
PC12 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). RPMI1640, penicillin, streptomycin, fetal bovine serum (FBS), horse serum (HS), and nerve growth factor (NGF) were purchased from Invitrogen (Carlsbad, CA, USA). PC12 cells were grown in RPMI1640 with 5% FBS, 10% HS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin at 37°C in 5% CO2 humidified air.
To study neurite outgrowth, medium was changed to RPMI containing 1% HS, 100 ng/mL of NGF, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. Cells were further cultured for 5 days. Cells with at least one neurite longer than two-body lengths were counted as neurite-positive. At least 500 cells were counted for each group performed in triplicate.
Cell viability was determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma, St Louis, MO, USA) assay. MTT in PBS was added to cells at a final concentration of 0.5 mg/mL. After 1 hr of incubation at 37°C, the medium was aspirated and 100 μL of DMSO (Sigma, St Louis, MO, USA) was added to dissolve cells, after which absorbance was measured at 570 nm.
Western blot analysis was carried out as described previously [17]. Membranes were immunoblotted with primary specific mouse monoclonal antibodies for Bax, parkin, p21, p53, cyclinD1, and β-actin (1:500 dilution, Santa Cruz Biotechnology Inc.). Blot was then incubated with corresponding conjugated anti-rabbit and anti-mouse immunoglobulin G-horseradish peroxidase (1:2000 dilution, Santa Cruz Biotechnology Inc.). Immunoreactive proteins were detected with an ECL Western blotting detection system. Relative densities of protein bands were scanned by densitometry using MyImage (SLB, Seoul, Korea) and quantified by Labworks 4.0 software (UVP Inc., Upland, CA, USA).
Data were analyzed using GraphPad Prism 4 ver. 4.03 software (GraphPad Software, La Jolla, CA). Data are presented as the mean ± S.D. Differences in data were assessed by one-way analysis of variance (ANOVA). When the P value in the ANOVA test indicated statistical significance, differences were assessed by Dunnett’s test. A value of P<0.05 was considered to be statistically significant.
Results
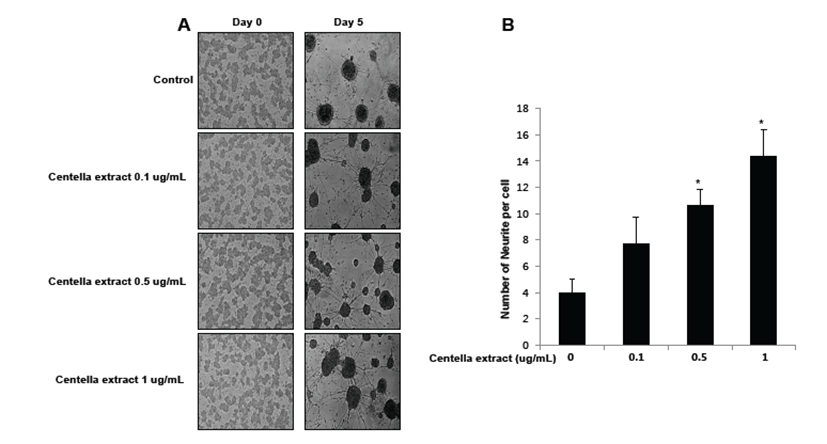
To study the effect of Centella asiatica on differentiation of neural stem cells, we cultured primary neural stem cells isolated from the cortex of E18.5 mouse embryos. Neural stem cells (NSCs) were allowed to differentiate in the presence of Centella asiatica for 5 days. Increased neurite outgrowth was observed in cells treated with Centella asiatica as compared to the control (Fig. 2A). We quantitatively analyzed neurites of neural stem cells (Fig. 2B). Cells treated with Centella asiatica showed significantly longer primary and secondary neurites as well as a greater number of neurites per cell as compared to the control. Thus, Centella asiatica promoted neurite outgrowth, indicative of differentiation, as well as branching of foetal NSCs.
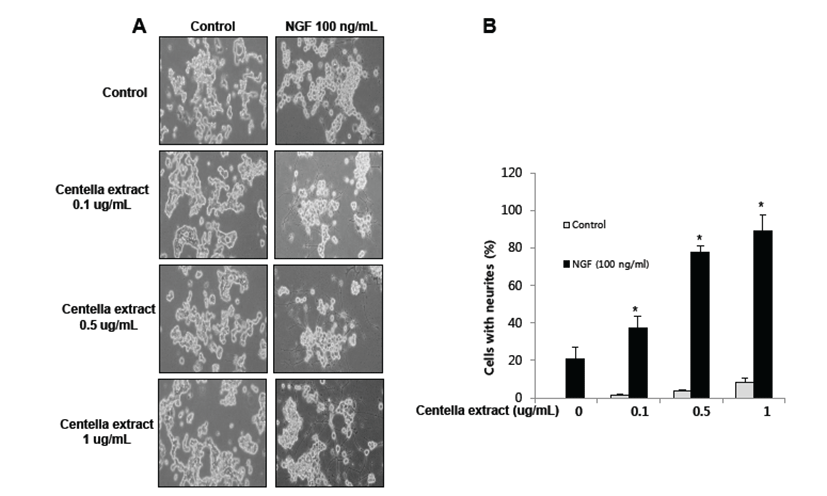
PC12 cells have previously been used as an instructive model for studying the underlying mechanisms of neuronal differentiation in response to NGF [18]. To determine whether or not Centella asiatica has a similar effect in non-stem cells, PC12 cells were differentiated for 5 days with Centella asiatica following stimulation with NGF (100 ng/mL). We showed that neurite outgrowth and branching of PC12 cells were stimulated by NGF treatment, and this effect was enhanced by Centella asiatica (Fig. 3A). For quantified data, average number of neurites per cell was much higher in Centella asiatica-treated cells as compared to control cells (Fig. 3B). Thus, Centella asiatica promoted neurite outgrowth both in PC12 cells as well as neural stem cells.
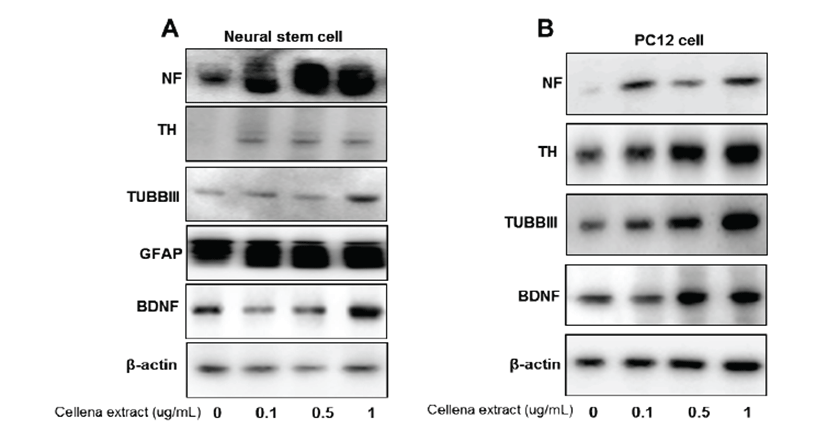
To understand the contribution of Centella asiatica towards specific neural cell types, neural stem cells were differentiated into various neural subtypes, followed by Western blotting with lineage-specific antibody markers (Fig. 4A). Expression of tubulin-III (TUBBIII), which is a neuronal cell marker [19], as well as tyrosine hydroxylase (TH), which is a dopaminergic neuronal cell marker protein [20], was elevated by Centella asiatica treatment. Moreover, expression of neurofilament (NF), which is a major component of the neuronal cytoskeleton that functions primarily to provide structural support for the axon and regulate axon diameter in neurons [21], was elevated by treatment with Centella asiatica. Expression of glial fibrillary acidic protein (GFAP), which is an astrocyte specific marker [22], was also elevated by treatment of Centella asiatica. Moreover, we measured expression of brain-derived neurotrophic factor (BDNF), which acts on certain neurons of the central and peripheral nervous systems, helps support survival of existing neurons, and encourages growth and differentiation of new neurons and synapses [23]. In the brain, BDNF is vital to learning, long-term memory, and higher thinking [24]. Expression of BDNF was also enhanced by Centella asiatica. In PC12 cells, expression of TUBBIII, TH, NF, and BDNF was elevated by Centella asiatica (Fig. 4B), which is similar to the pattern in neural stem cells. From these results, Centella asiatica has a promoting effect on neurogenesis.
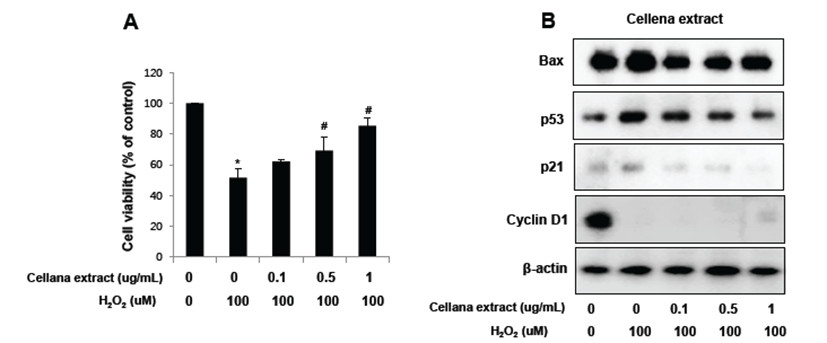
Neuroprotective activity of Centella asiatica was further tested in cultures of noradrenergic pheochromocytoma PC12 cells challenged by exposure to 100 μM of H2O2 for 8 hr, followed by treatment with Centella asiatica for 24 hr. Cell growth was quantitatively analyzed by MTT assay, whereas cell death was analyzed by Western blotting according to apoptosis-related proteins such as p53 and bax as well as cell cycle-related proteins such as p21 and cyclin D1. Centella asiatica significantly reduced H2O2-induced cell death. Expression of apoptosis-related proteins such as p53 and bax as well as cell cycle inhibitory protein p21 was up-regulated by H2O2 treatment but inhibited by Centella asiatica. Moreover, expression of cell cycle-related protein cyclin D1 was highly expressed in PC12 cells but inhibited by H2O2 treatment. On the other hand, expression pattern of cyclin D1 was reversed after treatment with Centella asiatica. From these results, we suggest that Centella asiatica inhibits oxidative stress-induced neural cell damage through regulation of apoptosis- and cell cycle-related proteins. Fig. 5
Discussion
Here, we investigated Centella asiatica leaf extracts as potent inducers of neurogenesis, neuronal differentiation, and neurite outgrowth.
Centella asiatica contains large amounts of pentacyclic triterpenoids, including asiaticoside, brahmoside, asiatic acid, and brahmic acid also known as madecassic acid, as well as centellose, centelloside, and madecassoside [25]. Several studies have demonstrated that triterpenoids regulate neurogenesis. For example, oleanolic acid, which is a pentacyclic triterpenoid, is abundantly present in a variety of plants and medicinal herbs such as Olea europaea, Viscum album L., and Ligustrum lucidum and induces differentiation of NSCs to neurons via a Nkx-2.5-dependent mechanism [26]. Suh’s group also showed that the new synthetic oleanane triterpenoid 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO) enhances neuronal differentiation of rat PC12 pheochromocytoma cells induced by nerve growth factor [27]. Another group suggested that triterpenoids from Panax notoginseng could enhance neurite outgrowth of NGF-mediated PC12 cells [28]. Ginsenosides, which are component of ginseng and a special type of triterpenoid saponins, have effects on neuronal differentiation of mouse embryonic stem cells through a GR-dependent signaling pathway [29]. From these studies, triterpenoids could have effects on neurogenesis. We hypothesize that Centella asiatica, which contains large amounts of pentacyclic triterpenoids, may promote neurogenesis. Similar to other studies, in our study, Centella asiatica promoted differentiation of neural stem cells as well as neurite outgrowth in PC12 cells.
Based on previously reported investigations on triterpenoid-containing compounds, further neuroprotective activities are needed. Recent data suggested that the novel triterpenoid CDDO-Im (2-cyano-3,12 dioxooleana-1,9 dien-28-oyl imidazoline) protects neurons against ischemic injury through up-regulation of heme oxygenase-1 [30] . Moreover, the synthetic triterpenod CDDO-Me limits production and secretion of neurotoxic pro-inflammatory cytokines, attenuates intracellular ROS accumulation, and confers neuroprotection in vivo [31]. Acetyl-11-β-boswellic acid (AKBA), which is an active triterpenoid from Boswellia serrata extract, has protective effects in neuronal cells against cerebral ischemic injury via the Nrf2/heme oxygenase-1-dependent pathway [32]. The neuroprotective properties of pentacyclic triterpenoids have attracted increased attention recently. Oleanolic acid also has protective effects against cerebral ischemic damage and H2O2-induced injury in vitro [26]. Recently, ursolic acid, a naturally occurring pentacyclic triterpenoid, was shown to promote neuroprotection after cerebral ischemia in mice by activating the Nrf2 pathway [33]. Another study revealed that AKBA may have a stronger antioxidant effect than ursolic acid in mice [32]. Similar to other studies, Centella asiatica also protected neuronal cells from oxidative stress. Neuroprotective activity of Centella asiatica was also observed in noradrenergic pheochromocytoma PC12 cell. Finally, Centella asiatica inhibited oxidative stress-induced neural cell damage through regulation of apoptosis- and cell cycle-related proteins.
During central nervous system development, neuronal stem cells proliferate and then differentiate into neuron and glial cells [34]. In this study, we measured expression of neural cell lineage markers to demonstrate whether or not Centella asiatica promotes neuronal differentiation of neural stem cells. Expression of the neuronal cell marker TUBBIII, the dopaminergic neuronal cell marker protein, TH, and the astrocyte-specific marker GFAP increased upon Centella asiatica treatment. Moreover, expression of NF, a major component of the neuronal cytoskeleton that functions primarily to provide structural support for axons and regulate axon diameter in neurons, also increased after Centella asiatica treatment. Nervous system function depends on the complex architecture of neuronal networks, which arise from the morphological intricacy that neurons acquire during the course of differentiation [35]. This process of differentiation is regulated by a variety of signaling mechanisms, including growth factors, cytokines, transcription factors, and soluble as well as membrane-bound receptors [36]. Accordingly, we measured expression of BDNF since it acts on certain neurons in the central and peripheral nervous systems, supports survival of existing neurons, and encourages growth and differentiation of new neurons and synapses [18]. Expression of BDNF was also enhanced by Centella asiatica treatment. From these results, we suggest that Centella asiatica has a promoting effect on neurogenesis.
In conclusion, extract of Centella asiatica leaves induces differentiation of neural precursor cells (NPCs) into neuronal cells, and this may be the mechanism underlying the neuroprotective effect of Centella asiatica.