Introduction
Insulin is a peptide hormone secreted from pancreatic beta cells. This hormone maintains the glucose level in the blood stream and thus supplies energy source to the cells. The condition of insulin deficiency or resistance called diabetes mellitus (DM) results in accumulation of circulating glucose, accelerating hepatic glycogenolysis and gluconeogenesis, while the cells of the body are starved [1, 2]. These conditions usually cause an osmotic diuresis owing to excessive renal threshold of glucose reabsorption, compensatory polydipsia, weight loss, and polyphagia [3-7]. There are two types of DM–insulin-dependent and non-insulin-dependent. In canine DM, most patients have the insulin-dependent type DM requiring lifelong exogenous insulin [8, 9].
The exogenous insulin preparations are typically categorized as short-acting, intermediate-acting, and long-acting basal insulins. Short- and intermediate-acting insulins can decrease the blood glucose concentration rapidly; they are useful for treating diabetic ketoacidosis (DKA). On the other hand, they are less preferred as a home treatment because of their requirement for frequent administrations [3]. Recently, insulin detemir, a long acting insulin analogue, was introduced in veterinary medicine. This insulin has the B29 positioned lysine amino acid bound to a 14-carbon fatty acid and is without the B30 threonine [3, 10, 11]. Slow dissociation of insulin molecules and binding to albumin in subcutaneous tissues are thought to be the reasons for the prolonged action of insulin detemir. The albumin-insulin complex also contributes to the slower distribution of insulin detemir to target tissues [12].
Unfortunately, published studies of insulin detemir in dogs are still limited. Therefore, we selected 6 dogs with insulin-dependent DM that were being treated with insulin detemir. The trends of their blood glucose concentrations were investigated during hospitalizations. In addition, we evaluated the initial and following doses of insulin detemir for several months.
Materials and Methods
For this study, 6 canine patients with insulin-dependent DM were selected from the dogs referred to us from 2013 to 2014. We included only the dogs which were being treated with insulin detemir at the time of the writing of this report and of which owners willingly brought more than twice for monthly checks of the blood glucose levels. The patient information of the present study is shown in Table 1. The mean ± standard deviation (S.D.) age of onset in the 6 dogs was 8.8 ± 1.72 years (range, 7 to 11 years). Fasting glucose concentrations of all the dogs were higher than 440 mg/dL. DM in 2 dogs had been poorly controlled with other types of insulin analogues such as Neutral Protamine Hagedorn (NPH) or insulin glargine, while the remaining 4 dogs were newly diagnosed with canine DM.
All patients suspected as having DM were evaluated by physical examination, laboratory tests, canine pancreatic lipase immunoreactivity (c-PLI) ELISA kit test (Canine SNAP cPL, IDEXX Laboratories, Westbrook, Maine, USA),radiography, and ultrasonography. Hyperadrenocorticism was diagnosed by checking the blood cortisol concentration (SNAP® Cortisol Test, IDEXX Laboratories, Westbrook, Maine, USA) before and after the adrenocorticotropic hormone (ACTH) stimulation tests. The patients presented the typical clinical signs of DM such as polyuria, polydipsia, or weight loss. The concurrent diseases diagnosed in each dog were presented in Table 1.
Once the dogs were diagnosed with DM, they were hospitalized for at least 3 days until we were able to obtain stable glucose curves repeatedly (nadirs<155 mg/dL) and the clinical signs of diabetes disappeared. Blood glucose concentrations were checked every hour and urine glucose and ketone levels were detected with urine sticks (Uriscan® 10 SGL, YD diagnostics, Yongin, Korea).
Three dogs with DKA were initially treated with intravenous fluid and regular human insulin until urine ketone was not detected (Table 1). Insulin detemir (Levemir®, Novo Nordisk, Bagsvaerd, Denmark) was administered subcutaneously in all dogs every 12 hours after the canine patients were fed commercial dog food (W/D, Hills, USA). The dogs diagnosed with hyperadrenocorticism were well treated with trilostane (Vetoryl®, Dechra Veterinary Products Ltd, Shrewsbury, UK) administration based on regularly checked levels of blood cortisol (Table 1).
After discharge from the hospital, the dogs were managed with insulin detemir and the diabetic feed twice a day at home. General condition such as body weight, appetite, and relapse of polyuria/polydipsia were regularly monitored by the owners at home. About 1 month after the first prescription of insulin detemir, the blood glucose curves including the nadir levels were rechecked in all dogs. Blood glucose level curves spanning a duration of over 12 hours could not be checked in all the dogs during follow-up visits because of the owners’ refusals. The doses of insulin detemir in each case were regulated based on the nadir levels of the blood glucose.
We analyzed the hourly blood glucose concentrations and their nadir levels of each dog and their mean values ± S.D. on the last day of hospitalization. The mean doses ± S.D. of insulin detemir were also evaluated in each dog during the follow-up period. The differences rates between the initial doses and the changed doses after 1 month were assessed. All data were analyzed with SPSS (version 12.0 KO) softwares.
Results
At least 4 cycles of blood glucose concentrations were evaluated after the first administration of insulin detemir in all dogs. The stable blood glucose curves of every dog were obtained on the last day of hospitalization. Hourly blood glucose concentrations in each dog are described in Fig. 1. The mean levels of total blood glucose over a duration of 12 hours in each dog ranged from 113.5 to 327.3 mg/dL (Table 2). The nadir levels of blood glucose were recorded at 3 hours (2 dogs), 4 hours (1 dog), 7 hours (1 dog), and 9 hours (2 dogs) after insulin detemir administrations (Fig. 1). The mean ± S.D. of glucose nadirs in the dogs was 103 ± 33 mg/dL (range, 69 to 155 mg/dL).
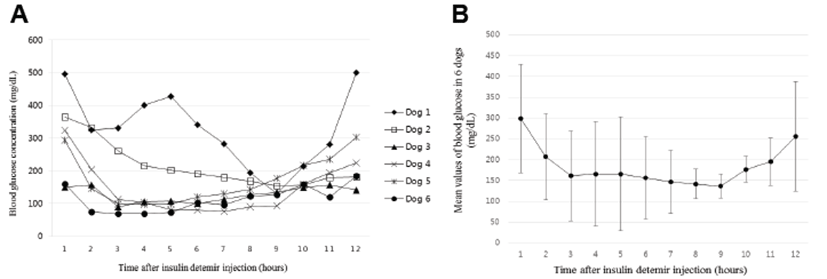
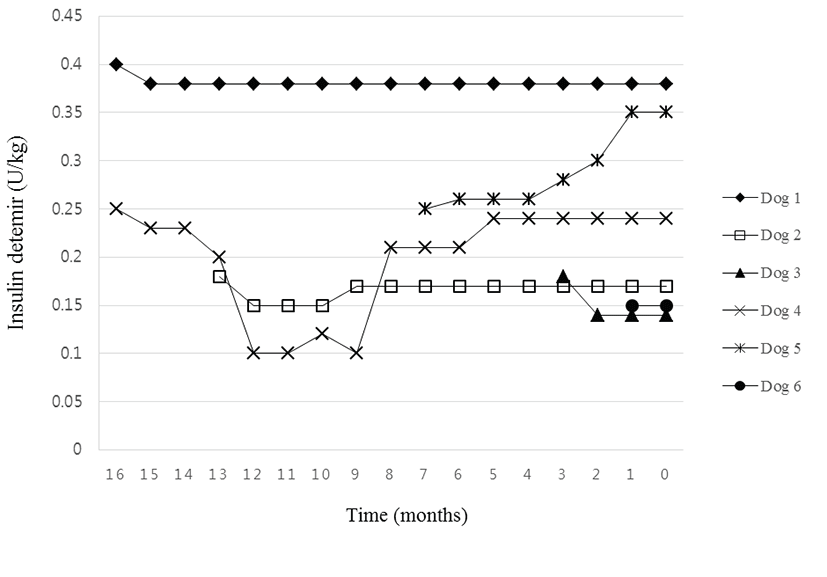
The initial doses of insulin detemir for stable blood glucose curves in the 6 dogs are presented in Table 2. The range of the initial doses was between 0.15 and 0.4 U/kg (mean dose ± S.D., 0.24 ± 0.09 U/kg). Insulin detemir doses were regulated based on the results of the monthly follow-up evaluations. The patterns of the insulin doses in each dog throughout this study are shown in Fig. 2. The mean insulin doses of each dog in the follow-up periods are shown in Table 2. After the first follow-up check, the doses of insulin detemir were decreased in 4 dogs, increased in 1 dog, and maintained in 1 dog. The changes in insulin doses after 1 month are shown in Table 2. The mean difference in doses between the initial and the first following month was 0.017 ± 0.019 U/kg (range, –0.01 to 0.04 U/kg). The rates of differences in the insulin doses are also presented in Table 2.
Discussion
The evaluations of blood glucose concentrations over 12 hours in 6 diabetic dogs showed that, except in 1 dog, the mean glucose concentrations were under 250 mg/dL and were within the ideal range (between 100 and 250 mg/dL) suggested by a previous study [8, 13]. In this study, not all dogs showed severe hypoglycemia (<60 mg/L) and the Somogyi effect during disease management. Post-prandial hyperglycemia (>200 mg/kg), however, was seen in 4 of the 6 dogs. Insulin detemir is known as peakless or flatter acting insulin, which could gently rise and fall the blood glucose levels [14, 15]. One veterinary study also reported a similar feature of insulin detemir in dogs (n=5) where the highest blood glucose level was under 200 mg/dL [16]. In most of the dogs included in this study, this “peak-less” effect of insulin detemir may result in a stable blood glucose concentration for a longer duration. The peak effect of insulin detemir, however, was revealed in glucose curves of 2 dogs. The exact cause of the observed peak effect is not clear, but the existence of concurrent diseases, the stressful hospital environment, or other individual factors may have influenced the glucose curves. Further study is required to clarify why the peak effect of insulin detemir occurs in some dogs.
Two dogs in our study had been poorly controlled with other types of insulin analogues such as NPH and glargine even though their concurrent disease had been well controlled. After changing to insulin detemir, the owners of these 2 dogs were satisfied with the efficiency and convenience of insulin detemir. Insulin detemir is convenient to use at home because it is long acting and therefore, usually needs to be injected only once or twice a day in dogs [16]. One of the two dogs had previously shown irregular cycles of blood glucose with NPH. After changing to insulin detemir, the dog had regular and stable 12-hour cycles of blood glucose during the follow-up period. Similarly, the other dog showed poor control with insulin glargine. In this dog, the initial dose of insulin glargine was 0.35 U/kg, but the following doses were increased rapidly up to 0.9 U/kg for 1 month due to poor glycemic control. After changing to insulin detemir, the dose of insulin was stable (0.38 to 0.4 U/kg) for 17 months. In a previous study, 13 diabetic dogs including 10 dogs showing poor treatment with NPH and insulin glargine were managed with insulin detemir and showed similar results to the present study; hyperglycemia in these dogs was well controlled for approximately 10 months [3]. Another study suggested that the difference of the potencies between insulin detemir and others might be related to the superior ability of insulin detemir in controlling glucose concentration [16]. Insulin detemir is produced at a 4-times higher molar concentration (24 26mmol/L) than other insulin products such as NPH and glargine, which are produced at 6 mmol/L. Regarding the stability of insulin detemir, several studies in humans have suggested that insulin detemir provides an overall similar blood glucose control [17-21]. The current study also showed regular and stable 12-hour cycles of blood glucose level throughout the follow-up period using insulin detemir in the 2 dogs poorly controlled previously with other insulins as well as in the other 4 dogs newly diagnosed.
The nadir levels of the blood glucose and serum fructosamine concentrations were measured when poor glucose control was suspected during the follow-up period. In cases in which the owners were willing to let the dogs stay in the hospital after the follow-up visits, entire 12-hour glucose curves were obtained. Based on the results, we modified the doses of insulin detemir. The doses were modified in 5 of the 6 dogs after the first follow-up visits. The dose levels were adjusted by a decrease of 4% to 23% in 4 dogs and an 8% increase in 1 dog. In other words, hypoglycemia occurred in 4 of the 6 dogs (67%) after 1 month. This might be due to temporary glycemic changes when the dogs were stressed under an environment of long-term hospitalization [22]. Therefore, when insulin detemir is administered initially, the veterinarian should warn the owners of possible hypoglycemia even at home. If needed, the lower than ideal doses obtained during the hospitalizations might be helpful in preventing hypoglycemia. The overall changes in the doses in our study were not variable. The mean doses in the 6 dogs ranged from 0.15 to 0.38 U/kg (the maximum dose was 0.4 IU/kg, and the minimum was 0.1 IU/kg; the overall average was 0.25 ± 0.1 U/kg). A previous study suggested 0.15 ± 0.07 U/kg as the optimal dose of insulin detemir in dogs [16]. Another study claimed the required doses of insulin detemir in canine patients with diabetes to be 0.2 U/kg (by Ford et al.) and 0.3 U/kg (range, 0.1 to 0.6 U/kg) (by the Veterinary Hospital of University of California, Davis) [3]. There is no optimized protocol for the use of this new insulin analogue in canine patients with DM. Additionally, the required insulin dose may be variable based on concurrent disease, severity of hyperglycemia , and individual factors. Further studies are needed to establish an optimum protocol for the use of insulin detemir in veterinary medicine.
In the present study, we evaluated the trends of blood glucose concentrations during hospitalizations and changes in the doses of insulin detemir for several months in 6 diabetic dogs. Our results indicate that insulin detemir is useful and stable medication for twice a day home therapy in diabetic dogs for several months. Canine diabetic patients who had concurrent diseases or a history of poor control with other types of insulin could be managed effectively with insulin detemir, as well. The results of this study suggest insulin detemir to be of value as an alternative choice against the traditional insulins for the poorly controlled patients and also as a home therapy medication in canine DM. The limitations of this study are the small sample size and the short follow-up period in some of the dogs. However, this study might help inform veterinary practitioners regarding the use of insulin detemir for canine insulin-dependent DM. Insulin detemir was introduced recently in veterinary medicine, and there are only limited studies in dogs. The complications of insulin detemir are not clear in canine patients. Moreover, a long-term evaluation of insulin detemir (over several years) in veterinary medicine is required. Therefore, further studies are needed to deal with these limitations of insulin detemir for the ideal management for diabetic animals.