ARTICLE
Prevalence of serum allergen-specific immunoglobulin E for canine atopic dermatitis in Korea
Hyo-Mi Jang1,#, Gwi-Seon Yeo1,#, Ji-Hyun Kim1, Cheol-Yong Hwang2, Jae-Eun Hyun2, Soon-Shin Kim3, Yang-Ho Kang4, Dong-In Jung1,*
These authors contributed equally to this study.
*Corresponding author: Dong-In Jung, College of Veterinary Medicine, Gyeongsang National University, Jinju 660-701, Korea Tel: +82-55-772-2361, E-mail:
jungdi@gnu.ac.kr
© Research Institute of Veterinary Medicine. This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: Nov 17, 2014; Revised: Dec 11, 2014; Accepted: Dec 12, 2014
Abstract
Canine atopic dermatitis (CAD) is an allergic skin disease with characteristic clinical features associated with immunoglobulin E (IgE) antibodies. Identification of the causative allergens is the diagnostic goal, which is essential to treat and manage CAD patients. CAD is commonly associated with environmental allergens surrounding the patients. For this reason, it is important for diagnostic tests to select allergens that are related to the environment of each country and each province. There are two main allergen-specific tests, serological IgE test (SAT) and intradermal skin test (IDT). SAT did not show direct cutaneous reaction but did show serological reaction against allergens. However, SAT is simpler and more convenient than IDT in small animal practice. In this study, we selected domestically prevalent allergens for SAT, including 60 food allergens and 60 inhalant allergens, and tested eight dogs tentatively diagnosed with CAD based on Favrot’s criteria. Furthermore, IDT was performed on four dogs from the SAT group for comparison of SAT and IDT, and the results were very similar. In SAT, four types of mites (Bloomia tropicalis, Glycophagus domesticus, Euroglyphus maynei, and mite mixture 1 Korea; house dust mites), four types of molds (Botrytis cinerea, Alternaria alternata, mold fungi mixture 11, mold fungi mixture), and one type of pollen (tree pollen mix 3 Korea) induced a reaction in more than half of dogs tested. In IDT, all four dogs reacted positively to Dermatophagoides farinae, and three reacted positively to Dermatophagoides pteronyssinus and house dust. The mean agreement rate between SAT and IDT in this study was 76.3%. This is the first trial to apply local allergens for SAT in Korean veterinary medicine, and it might play an important role for diagnoses and management of animal allergic diseases.
Keywords: atopic dermatitis; serum allergen-specific IgE test; intradermal skin test; dog; Korea
Introduction
The definition of canine atopic dermatitis (CAD) is genetically predisposed inflammation and pruritic skin disease with characteristic clinical features associated with immunoglobulin E (IgE) antibodies most commonly directed against environmental allergens [1]. Since there is currently no definitive diagnostic test for CAD, veterinary diagnosis is based on the evaluation of clinical symptoms and the exclusion of other potential causes [2, 3, 4].
Once a tentative diagnosis of CAD is made based on clinical criteria, testing for allergen-specific IgE can help to confirm the diagnosis. The most common methods of allergen-specific testing in veterinary medicine are the intradermal skin test (IDT) and the serum allergen-specific IgE test (SAT). IDT is widely accepted as the gold-standard technique for allergic skin diseases [5]. Nonetheless, IDT is limited by the requirement for anesthesia, shaving, and the withdrawal of medication that may influence allergic reactions [5]. Consequently, SAT is used more frequently than IDT because of its convenience: only a small volume of animal serum is required for testing and withdrawal of anti-allergy drugs is not necessary [6]. It is essential to identify the specific environmental allergens in domestic region and residential area for allergen-specific IgE tests. Unfortunately, there is no commercially available SAT based on specific geographical allergens for dogs in Korea. In this study, 120 common allergens in Korea were designed for use in a canine SAT and applied to eight dogs with CAD. Four of them were also tested by IDT to compare the results between SAT and IDT.
Materials and Methods
Serum allergen-specific IgE test (SAT)
Eight dogs with dermatological problems were selected for testing in this study (Table 1). The dogs were tentatively diagnosed with CAD based on the two sets of diagnostic criteria proposed by Favrot et al (Table 2) [2].
Table 1.
Signalments and results of diagnostic examinations in 8 dogs
|
No. of Dogs |
Signalments
|
Results of diagnostic examinations
|
|
Breed |
Age |
Sex |
Total IgE kit test |
Set 1 [2] |
Set 2 [2] |
|
|
Dog 1 |
French Bulldog |
1-year-old |
Intact female |
Positive* |
6/8 |
6/7 |
|
Dog 2 |
Cavalier King Charles Spaniel |
2-year-old |
Castrated male |
Positive* |
7/8 |
6/7 |
|
Dog 3 |
Mongrel |
2-year-old |
Spayed female |
Positive* |
6/8 |
4/7 |
|
Dog 4 |
Maltese |
5-year-old |
Castrated male |
Positive* |
6/8 |
6/7 |
|
Dog 5 |
Old English Sheepdog |
11-year-old |
Spayed female |
Positive† |
8/8 |
7/7 |
|
Dog 6 |
Shih Tzu |
11-year-old |
Castrated male |
Positive† |
6/8 |
5/7 |
|
Dog 7 |
Shih Tzu |
2-year-old |
Castrated male |
Positive† |
7/8 |
7/7 |
|
Dog 8 |
Pekingese |
3-year-old |
Intact female |
Positive† |
6/8 |
5/7 |
Download Excel Table
Table 2.
Diagnostic criteria for canine atopic dermatitis [2]
|
Set 1 |
Set 2 |
|
|
1. Age on set <3 years |
1. Age on set <3 years |
|
2. Mostly indoor |
2. Mostly indoor |
|
3. Corticosteroid-responsive pruritus |
3. pruritus with no visible lesions at onset |
|
4. Chronic or recurrent yeast infections |
4. Affected front feet |
|
5. Affected front feet |
5. Affected ear pinnae |
|
6. Affected ear pinnae |
6. Non-affected ear margin |
|
7. Non-affected ear margin |
7. Non-affected dorso-lumbar area |
|
8. Non-affected dorso-lumbar area |
|
Download Excel Table
Despite failing to meet the criteria, however, dog 3 did display the three key observations [5]. Exclusion of differential diagnoses for CAD signs and symptoms was made based on analysis of medical history. Prior to SAT testing, total IgE was established for each dog using a commercially available total IgE ELISA test kits (Allercept® E-screen 2G, Heska Corp., Fort Collins, USA and Asan Easy Test Canine IgE®, Asan Parm. Corp., Hwaseong-si, Korea; Table 1). At least 1.2 mL of serum sample was collected from each dog. Four panels of 120 allergens were used for detecting serum allergen-specific IgE (Alleisa Screen™, MEDIWISS analytic, Moers, Germany). Each panel consisted of 30 selected allergens commonly existed in Korea. 60 types of food allergens were included in the panels 1 and 2 (Table 3 and 4), while 60 types of inhalant allergens were in the panels 3 and 4 (Table 5 and 6). IgE concentration was quantified colorimetrically using a BLOTrix Reader scanning system (Bioscitec GmbH, Frankfurt, Germany).
Table 3.
List of allergens on panel 1 in this study
|
Code |
English |
|
|
F4 |
Wheat flour |
|
F49 |
Apple |
|
F92 |
Banana |
|
F244 |
Cucumber |
|
F27 |
Beef |
|
F31 |
Carrot |
|
F78 |
Caseine |
|
F83 |
Chicken |
|
F3_F23 |
Cod fish/ Crab mix |
|
F23_F24 |
Crab/ Shrimp |
|
F475 |
Duck |
|
F1 |
Egg white |
|
F75 |
Egg yolk |
|
FX3RB |
Fish mix (cod, shrimp, salmon, blue mussel and tuna) |
|
F79 |
Gluten |
|
F300 |
Goat milk |
|
F504 |
Goose |
|
NX_KO |
Nut mixture (cashew nuts, coconut and peanuts) |
|
F84 |
Kiwi |
|
F334 |
Lactoferrin |
|
F88 |
Lamb meat |
|
F2 |
Milk |
|
F457 |
Mushrooms mixed |
|
F33 |
Orange |
|
F26 |
Pork |
|
F502 |
Pork Liver |
|
F35 |
Potato |
|
F225 |
Potato |
|
F213 |
Rabbit |
|
F92 |
Rice flour |
Download Excel Table
Table 4.
List of allergens on panel 2 in this study
|
Code |
English |
|
|
F41 |
Salmon |
|
F420 |
Sea Bass |
|
F24 |
Shrimps |
|
F14 |
Soy bean |
|
F258 |
Calamari |
|
F44 |
Strawberry |
|
F54 |
Sweet Potato |
|
F258 |
Tomato |
|
F204 |
Trout |
|
F40 |
Tuna |
|
F284 |
Turkey |
|
F329 |
Water melon |
|
F215 |
Lettuce |
|
F478 |
Tofu |
|
F291 |
Cauliflower |
|
F260 |
Broccoli |
|
F216 |
Cabbage |
|
FX400 |
Cheese mix |
|
F218 |
Pepper |
|
F288 |
Blueberry |
|
F315 |
Green Beans |
|
FX2 |
Meal mix |
|
F247 |
Honey |
|
F236 |
Whey |
|
F94 |
Pear |
|
F95 |
Peach |
|
F12 |
Pea/ Bean |
|
F465 |
Quail |
|
F114 |
Sunflower seed |
|
F2MP |
Milk powder |
Download Excel Table
Table 5.
List of allergens on panel 3 in this study
|
Code |
English |
|
|
E1 |
Cat epithel |
|
E2 |
Canis domesticus |
|
E5 |
Dog epithel |
|
E84_E74_73 |
Golden hamster/Mouse/Rat |
|
Gx9 |
Mixed grasses 9 (barley, reed, smooth brome and oat) |
|
Gx10 |
Mixed grasses 10 (orchard grass, timothy grass, meadow fescue grass, rye grass and kentucky grass) |
|
Getmix |
Cereals pollen mix (rye, oats, wheat, barley and maize) |
|
Gx11 |
Mixed grasses 11 (sweet-scanted vernal grass, reed, rye grass and wooly holcus) |
|
M6 |
Alternaria alternate |
|
M47 |
Aspergillus flavus |
|
MX11 |
Mould fungi mixture 11 (Asp. fumigatus, Asp. niger, Asp. amstelodami and Asp. nidulans) |
|
M7 |
Botrytis cinerea |
|
M5 |
Candida albicans |
|
M208 |
Chaetomium globosum |
|
M2 |
Cladosporium herbarum |
|
M17 |
Curvularia spicifera |
|
M14 |
Epicoccum purpurascens |
|
M9 |
Fusarium moniliforme |
|
M212 |
Micropolyspora faeni |
|
MX1TH |
Mould fungi mixture (Alt. alternate, Clad.herbarum, Clad. cladosporioides and Stemphylium botryosum) |
|
MX7 |
Mould fungi mixture 7 (Asp. fumigatus, Asp. niger,Asp. flavus and Asp. versicolor) |
|
M4mu |
Mucor mucedo |
|
M23 |
Neurospora sitophila |
|
MX12 |
Mould fungi mixture 12 (Penic. viridicatum, Penic. expansum, Penic. notatum and Penic. chrysogenum) |
|
M90 |
Pityrosporum ovale |
|
M12 |
Aureobasidium pullulans |
|
M11 |
Rhizopus nigricans |
|
M210 |
Sporobolomyces roseus |
|
M80 |
S. Aureus-Enterotoxin A |
|
M81 |
S. Aureus-Enterotoxin B |
Download Excel Table
Table 6.
List of allergens on panel 4 in this study
|
Code |
English |
|
|
M266 |
S. Aureus- TSS-Toxin 1 |
|
M10 |
Stemphylium botryosum |
|
M213 |
Thermoactinomyces vulgaris |
|
M12 |
Aureobasidium pullulans |
|
P4 |
Anisakis simplex |
|
O20_O21 |
Grassland Cut/Hayfield Cut |
|
O1 |
Cotton wool |
|
P1 |
Ascaris spec. |
|
D201 |
Blomia tropicalis |
|
D73 |
Glycyphagus domesticus |
|
D70 |
Acarus siro |
|
DX1_KO |
Mite mixture 1 Korea (D.p., D.f., D.m.) |
|
DM1 |
Environmental mix1 |
|
D74 |
Euroglyphus maynei |
|
HX |
House dust |
|
D71 |
Lepidoglyphus destruktor |
|
D72 |
Tyrophagus putrescentiae |
|
K74 |
Silk |
|
K82 |
Latex |
|
TX1_KO |
Tree pollen mix 1 Korea (alder, birch, oak and popular) |
|
TX2_KO |
Tree pollen mix 2 Korea (acacia, eucalyptus, mesquite and mulberry tree) |
|
W1_W2_W3 |
Ambrosia/Ragweed/Franseria |
|
TX3_KO |
Tree pollen mix 3 Korea (cedar, cypress and juniper/savin tree) |
|
WX1_KO |
Weed pollenmix 1 Korea (hibiscus, rape and soeerl) |
|
I52_I50 |
Aedes ssp./Culex ssp. |
|
I90 |
House fly (Musca) |
|
I3 |
Common wasp- venom |
|
I6X |
Cockroach-mix |
|
I9_I39_I53 |
Flour beetle/Wheat weevil/Black fly |
|
O207 |
Water flea |
Download Excel Table
Based on the concentrations of IgE measured, results were stratified into 6 classes: a mild positive reaction was designated as class 1 or 2, a moderate reaction as class 2 or 3, and a strong positive reaction as class 3 or above. Any samples in which the positive control did not produce at least a class 4 reaction were excluded from analysis.
Intradermal skin test (IDT)
Four dogs (Dogs 1~4 from the SAT testing group) were further evaluated by IDT for comparison with SAT results. Any medication with the potential to influence IDT results was withdrawn for 21 days prior to testing.
A total of 53 water-soluble allergen extracts was used for IDT (Lenoir, North Carolina, USA). Each was administered at a dose known not to cause hypersensitivity in normal dogs (100~1000 Protein nitrogen units/mL or 1:1000 weight/volume). Histamine phosphate (27.5 μg/mL) was used as a positive control; 0.9% saline containing 0.1% phenol was used as a negative control. A scale of − to +3, relative to the mean of positive and negative controls, was used to classify wheal size. The equal or smaller size than negative control was classified as -; if it was smaller than the mean value of the positive and negative controls but it was bigger than negative control, it was classified as 1+; a wheal larger than the mean value but smaller than the positive control was classified as +2; wheals larger than the positive control were classified as +3. Because some of the allergens tested differed between SAT and IDT, only the allergens that were included in both tests were analyzed and compared in this study.
Results
Serum allergen-specific IgE test (SAT)
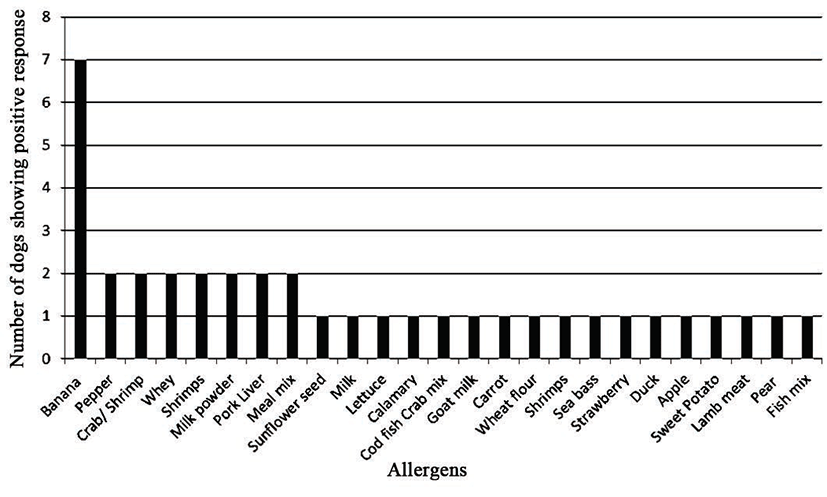
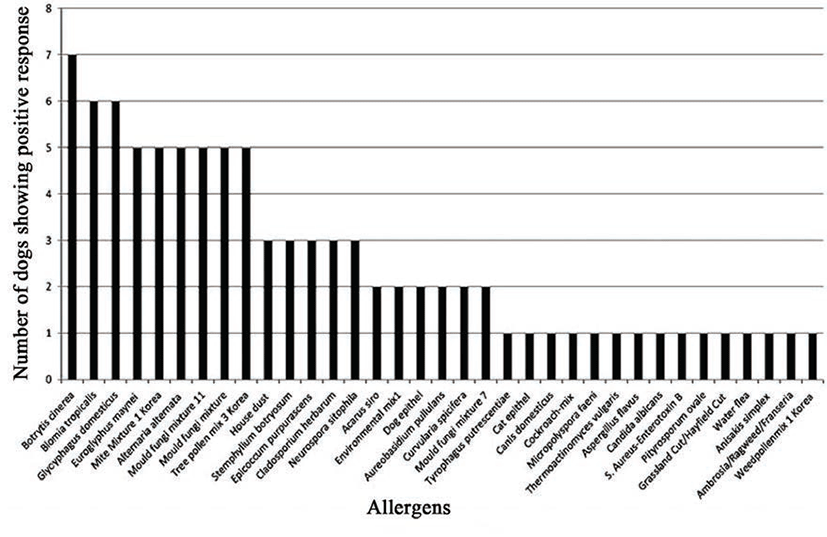
Based on the results obtained from panels 1 and 2 (common food allergens in Korea), banana was the most frequent positive allergen, stimulating a positive response in seven out of the eight dogs (87.5%; Fig. 1). On panels 3 and 4 (common inhalant allergens in Korea; Fig. 2), seven dogs (87.5%; Fig. 2) reacted positively to Botrytis cinerea (a fungal allergen) and six (75%; Fig. 2) reacted to Blomia tropicalis and Glycyphagus domesticus (mite allergens). Other allergens, such as Euroglyphus maynei, mite mixture 1 Korea, Alternaria alternate, mold fungi mixture 11, mold fungi mixture, and tree pollen mix 3 Korea produced a positive reaction in five of the eight dogs (62.5%; Fig. 2).
IDT Results and Comparison with SAT
In dog 1, positive reaction to house dust mites was indicated in both tests. However, dog 1 reacted to tree pollen mix 3 Korea in SAT only and to house dust in IDT only.
In dog 2, positive reactions to Aspergillus flavus and cypress tree pollen were seen in both tests. Dog 2 showed 1 type of positive allergen in SAT alone and 8 types of allergens in IDT.
In dog 3, positive reactions to the house dust mite and the house dust mix were observed in both tests. Four different allergens caused a positive reaction in SAT alone: Aspergillus flavus, ambrosia-ragweed-franseria, cockroach mix, weed pollen mix 1 Korea, and mold fungi mix 11.
In dog 4, positive reaction to the house dust mite allergens and house dust mix was seen in both tests. Dog 4 reacted to mold fungi mix 11 and tree pollen mix 3 Korea in SAT alone.
Total agreement rates between SAT and IDT are described in Table 7.
Table 7.
The agreements between SAT and IDT in 4 dogs
|
Allergen |
SAT (+) IDT (+) |
SAT (-) IDT (-) |
SAT (+) IDT (-) |
SAT (-) IDT (+) |
|
|
Cat epithelia |
0/4 |
4/4 |
0/4 |
0/4 |
|
Mixed grasses 9 (Canarygrass, Reed) |
0/4 |
4/4 |
0/4 |
0/4 |
|
Mixed grasses 10 (Bluegrass, Kentucky/June, Orchard grass, Ryegrass, Perennial Timothy) |
0/4 |
4/4 |
0/4 |
0/4 |
|
Cereals pollen (Oats, Common/Cultivated) |
0/4 |
4/4 |
0/4 |
0/4 |
|
Mixed grasses 11 (Canarygrass, Reed, Ryegrass, Perennial, Sweet vernal grass) |
0/4 |
4/4 |
0/4 |
0/4 |
|
Mould fungi mixture 11 (Aspergillus spp.) |
2/4 |
0/4 |
0/4 |
2/4 |
|
Fusarium moniliforme |
0/4 |
2/4 |
1/4 |
1/4 |
|
Mucor spp. |
0/4 |
3/4 |
1/4 |
0/4 |
|
Mould fungi mixture 12 (Penicillium) |
0/4 |
4/4 |
0/4 |
0/4 |
|
Rhizopus spp. |
0/4 |
3/4 |
1/4 |
0/4 |
|
Mite Mixture 1 Korea (Dermatophagoides farinae, Dermatophagoides pteronyssinus) |
3/4 |
0/4 |
1/4 |
1/4 |
|
House dust |
2/4 |
0/4 |
0/4 |
0/4 |
|
Silk |
0/4 |
4/4 |
1/4 |
0/4 |
|
Tree pollen mix 1 Korea (Poplar, Lombardy Poplar, White Birch mix, Eastern oak mix) |
0/4 |
3/4 |
1/4 |
0/4 |
|
Tree pollen mix 2 Korea (Acacia Mulberry, Paper Mulberry, White) |
0/4 |
3/4 |
1/4 |
0/4 |
|
Ambrosia/Ragweed/Franseria |
0/4 |
2/4 |
0/4 |
1/4 |
|
Tree pollen mix 3 Korea (Cedar, Red Cypress, Bald) |
1/4 |
1/4 |
0/4 |
2/4 |
|
Weed pollen mix 1 Korea (Sheep/Red sorrel) |
0/4 |
2/4 |
1/4 |
1/4 |
|
Insects (Aedes ssp./Culex ssp., House fly, Common wasp- venom, Cockroach-mix, Flour beetle/Wheat weevil/Black fly) |
0/4 |
3/4 |
0/4 |
1/4 |
Download Excel Table
Discussion
The atopic disease is defined as genetically predisposed tendency to develop IgE-mediated allergy to environmental allergen [1]. The environmental allergens vary significantly across countries and regions, thus it is essential to establish the geographic causative allergens in CAD. Here we present the first use of allergens prevalent in Korea for allergy testing in eight cases of CAD. This research may represent a valuable resource for Korean veterinary medicine.
There is currently no definitive diagnostic test for CAD and thus diagnosis of CAD is based on clinical manifestations, medical history and the exclusion of other potential causes of similar clinical symptoms [7]. In this study, we evaluated atopic dermatitis in eight dogs by applying Favrot’s criteria [2], considered the most sensitive and specific clinical criteria in veterinary medicine [7]. According to Farvot’s criteria, when the patient meets more than 5 of 8 criteria in set 1, the sensitivity is 0.854 and the specificity is 0.791 for CAD. In set 2, the sensitivity is 0.772 and the specificity is 0.83 for CAD if more than 5 of 7 criteria are presented [2].
Allergen-specific IgE tests, such as SAT or IDT, could represent the next step towards a diagnostic method for CAD. Allergen-specific IgE tests should not be used for sole diagnostic methods of CAD, but for identification of allergens to avoid causative substances and to inform the design of immunotherapy regimens [7]. Several investigators have demonstrated the diagnostic value of both allergen-specific IgE tests for CAD, when detected allergens are used for immunotherapy or avoidance strategies [8, 9].
Currently, IDT is considered the gold-standard method for identifying causative allergens, since there is little evidence of a correlation between circulating serum IgE levels and cutaneous IgE reactivity in SAT [7]. Many studies have evaluated the agreement between SAT and IDT results and most have reported similar hypersensitivity reactions to at least some allergens in CAD [10, 11, 12, 13]. SAT may therefore have similar diagnostic value to IDT, being significantly more convenient, and is often preferred by veterinary practitioners.
In SAT of the present study, four types of mite (Bloomia tropicalis, Glycophagus domesticus, Euroglyphus maynei, and mite mixture 1 Korea; house dust mites), four types of mold (Botrytis cinerea, Alternaria alternata, mold fungi mixture 11, mold fungi mixture) and one type of pollen (tree pollen mix 3 Korea) promoted a reaction in more than half of dogs tested. In IDT, all four dogs reacted positively to Dermatophagoides farina and three reacted positively to Dermatophagoides pteronyssinus and house dust. The mean agreement rate between SAT and IDT in this study was 76.3% (Table 7).
According to the results of current study, banana was the most frequent positive food allergen, stimulating a positive response in seven out of the eight dogs (87.5%). Other food allergens were not detected in over half of the patients. The link between food allergens and CAD remains controversial. Classifications of food allergy and CAD have until now been completely segregated, but recently the theory that food allergens may play an important pro-inflammatory role in CAD is gaining traction [7]. In human medicine, 33% of infants and 38.7% of young children with atopic dermatitis also have a food allergy [14, 15]. Previous veterinary studies have similarly found that 30% of dogs with CAD have concurrent adverse food reactions and 13~30% of CAD patients also exhibit cutaneous adverse food reactions [16, 17]. According to one previous report [18], however, SAT showed very low sensitivity (6.7%) and high specificity (91.4%) in canine adverse food reaction study. The positive and negative predictive values were 15.4% and 80.7%, respectively [18]. These results indicated that a positive result of canine SAT in food allergy could not be very helpful. The negative result of SAT of food allergens demonstrates that those antigens are tolerated well [18], thus we could utilize the negative result data in practice. In the present study, we evaluated SAT of food allergens in dogs and results were described in Fig. 1. Because of low sensitivity, we suggested that SAT results of food allergens in this study should apply only as reference data.
One previous study [19] demonstrated allergens associated with CAD in 35 dogs using IDT with 42 types of Korean allergen extracts. In 2011, Kim et al [20] also tested 39 common Korean allergens in 58 dogs with CAD using IDT. Recently, investigations of SAT for 101 CAD dogs were performed in Korea with 92 inhalant and food allergens [21]. However, there has not been the trial to choose allergens based on domestic environment and also not report comparing the results between IDT and SAT for the same dogs in Korea. Comparing these previous reports with the current study, we demonstrate that the positive ratio of specific allergens showed similar results (Table 8).
Table 8.
Comparison of the allergens between the previous studies and this study.
|
Allergens |
2002 [18] |
2011 [19] |
2014 [20] |
This study |
|
|
House dust mite |
D. Farinae |
63% |
49.1% |
61.4% |
62.5% |
|
D. ptereronyssinus |
31% |
|
House dust |
6% |
54.5% |
55.2% |
37.5% |
|
Molds |
No data |
67.3% |
Reported only individual mold species |
62.5% |
Download Excel Table
Total IgE ELISA test kits were used to support a diagnosis of CAD in this study; serological total IgE testing is usually used as a screening method in human allergic disease. Several studies have reported that total serum IgE levels may be predictive of positive reactions in SAT, clinical severity, and diagnosis of allergic diseases, although their value as diagnostic tools in humans is limited by variation in responses across different races [22, 23]. In veterinary medicine, conversely, total IgE serological tests have been widely reported as unreliable, detecting no significant difference in total serum IgE levels between normal and atopic dogs [10]. Further studies are needed to evaluate the diagnostic value of total IgE levels relative to positive results of SAT in CAD.
This study represents the first evaluation of Korean CAD allergens by SAT which composed of selected Korean type 120 allergens and the first comparison of SAT with IDT in Korean veterinary medicine. The limitations of this study include a small sample size and the omission of a standard for comparison between SAT and IDT. Further study using larger cohorts and immunotherapy trials based on causative allergens detected by SAT will be necessary in the future.
REFERENCES
Halliwell R. Revised nomenclature for veterinary al¬lergy. Vet Immunol Immunopathol. 2006; 114 p. 207-208.

Favrot C, Steffan J, Seewald W, Picco F. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet Dermatol. 2010; 21 p. 23-31.

Prélaund P, Guaguere E, Alhaidaidari Z, Faivre N, Heripret D. Reevalution of diagnostic criteria of canine atopic dermatitis. Rev Med Vet. 1998; 149 p. 1057-1064.

Willemse T. Atopic skin-disease a review and a recon¬sideration of diagnostic-criteria. J Small Anim Pract. 1986; 27 p. 771-778.

Noli C, Foster A, Rosenkrantz W, Griffin CE. Diagnosis of canine atopic dermatitis Veterinary al¬lergy. Hoboken Wiley-Blackwell. 2014; p. 70-77.

Olivry T, Saridomichelakis M. Evidence-based guide¬lines for anti-allergic drug withdrawal times before allergen-specific intradermal and IgE serological tests in dogs. Vet Dermatol. 2013; 24 p. 225-e49.

Miller WH, Griffin CE, Campbell KL, Marsella R. Hypersensitivity disorders Muller and Kirk’s Small animal dermatology. 2013; 7th edSt. LouisElsevier Mosby p. 363-431.

Park S, Ohya F, Yamashita K, Nishifuji K, Iwasaki T. Comparison of response to immunotherapy by intrader¬mal skin test and antigen-specific IgE in canine atopy. J Vet Med Sci. 2000; 62 p. 983-988.

Foster AP, Littlewood JD, Webb P, Wood JLN, Rog¬ers K, Shaw SE. Comparison of intradermal and serum testing for allergen-specific IgE using a FceRIa-based assay in atopic dogs in the, UK. Vet Immunol Immuno pathol. 2003; 93 p. 51-60.

Hill PB, Moriello KA, DeBoer DJ. Concentrations of total serum IgE, IgA, and IgG in atopic and parasitized dogs. Vet Immunol Immunopathol. 1995; 44 p. 105-113.

Roque JB, O’Leary CA, Kyaw-Tanner M, Latter M, Mason K, Shipstone M, Vogelnest L, Duffy D. High allergen-specific serum immunoglobulin E levels in nonatopic West Highland white terriers. Vet Dermatol. 2011; 22 p. 257-266.

Mueller RS, Burrows A, Tsohalis J. Comparison of in¬tradermal testing and serum testing for allergen-specific IgE using monoclonal IgE antibodies in 84 atopic dogs. Aust Vet J. 1999; 77 p. 290-294.

Olivry T, Jackson HA, Murphy K, Tater KC, Roberts M. Evaluation of a point-of-care immunodot assay for predicting results of allergen-specific intradermal and immunoglobulin E serological tests. Vet Dermatol. 2005; 16 p. 117-120.

Burks A, James JM, Hiegel A, Wilson G, Wheeler JG, Jones SM, Zuerlein N. Atopic dermatitis and food hy-persensitivity reactions. J Pediatr. 1998; 132 p. 132-136.

Burks A, Mallory SB, Williams LW, Shirrell MA. Atop¬ic dermatitis clinical relevance of food hypersensitivity reactions. J Pediatr. 1988; 113 p. 447-451.

Carlotti DN, Remy I, Prost C. Food allergy in dogs and cats. A review and report of 43 cases. Vet Dermatol. 1990; 1 p. 55-62.

White SD. Food hypersensitivity in 30 dogs. J Am Vet Med Assoc. 1986; 188 p. 695-698.

Bethlehem S, Bexley J, Mueller RS. Patch testing and allergen-specific serum IgE and IgG antibodies in the diagnosis of canine adverse food reactions. Vet Immunol Immunopathol. 2012; 145 p. 582-589.

Youn HY, Kang HS, Bhang DH, Kim MK, Hwang CY, Han HR. Allergens Causing Atopic Diseases in Canine. J Vet Sci. 2002; 3 p. 335-341.

Kim HJ, Kang MH, Park HM. Common allergens of atopic dermatitis in dogs comparative findings based on intradermal tests. J Vet Sci. 2011; 12 p. 287-290.

Kang MH, Kim HJ, Jang HJ, Park HM. Sensitization rates of causative allergens in dogs with atopic derma¬tits detection of canine allergen-specific IgE. J Vet Sci. 2014E-pub Ahead of Print.

Mediaty A, Neuber K. Total and specific serum IgE decreases with age in patients with allergic rhinitis, asthma and insect allergy but not in patients with atopic dermatitis. Immun Ageing. 2005; 2 p. 1-6.

Jung SW, Oh EJ, Lee H, Kim YG, Kim SY, Kim YS, Park YJ. Usefulness of Total IgE in Predicting Positive Allergen Specific IgE Tests in Korean Subjects. Korean J Lab Med. 2010; 30 p. 660-667.