Introduction
Atrial septal defect (ASD) is a persistent communication between the two atria caused by a hole in the inter-atrial septum [10, 11, 17]. In this disease, any pathological changes that increase the pressure in the left ventricle can worsen the left-to-right shunt. The left-to-right shunt then increases the filling pressure of the right chambers (preload) and imposes the right ventricle to take out more blood than the left ventricle. This causes the constant overloading of the right chambers of the heart and pulmonary hypertension. ASD is usually occurred by congenital heart defect either as an isolated type or a compound type [6, 8]. Acquired ASD is rare in dogs but can be occurred by the inflammation of cardiac structures (e.g. bacterial endocarditis) and conditions causing severe left atrial pressure or volume overload (e.g. degenerative mitral valve disease) [13-16, 18, 19]. Degenerative mitral valve disease (DMVD) is caused by a myxomatous degenerative change in the subendocardial valve leaflets and chordae tendineae in middle-aged to elderly dogs and is a major cause of severe left atrial volume and pressure overload in dogs [1, 2]. In this disease, the mitral valve can not close properly due to degenerative changes in mitral valve and thus causes the abnormal leaking of blood from the left ventricle, through the mitral valve, and into the left atrium, when the left ventricle contracts (i.e. mitral regurgitation; 3, 4, 7). The most common cause of DMVD is mitral valve prolapse (MVP) caused by myxomatous degeneration in cusps of mitral valve [5, 20]. Although acquired ASD secondary to rupture of the atrial septum in dogs with DMVD has rarely been reported in dogs [12, 19], the presence of acquired ASD should be considered in dogs with advanced stage of DMVD, because sudden onset of acquired ASD can affect the progression of pre-existing condition and therapeutic response.
Case report
A 12-year-old spayed female Pomeranian (weighing 2.4 kg) was referred with primary complaints of acute dyspnea, cough, and lethargy. Physical examination on this dog found exertional respiration and loud left apical systolic (grade 4/6) murmur. The dog also had moderate periodontal disease and grade III patella luxation in both legs. Heart rate was ~120 (range: 99~156) beats per min. The systolic blood pressure of this dog measured by Doppler method (811B, Parks medical, USA) was ~120 mmHg. No particular abnormalities were found in complete blood cell count and serum biochemistry.
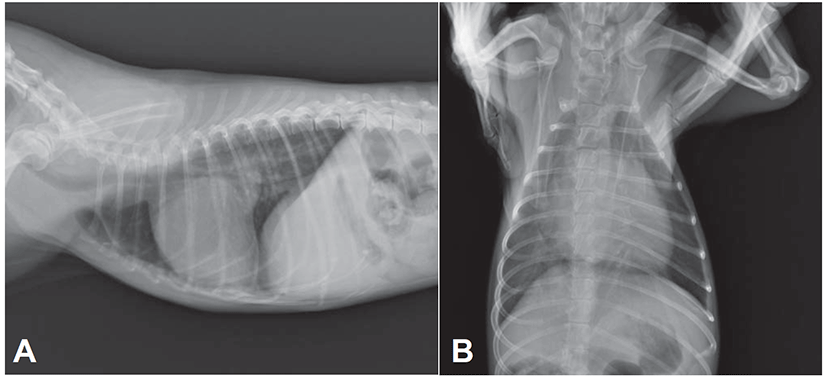
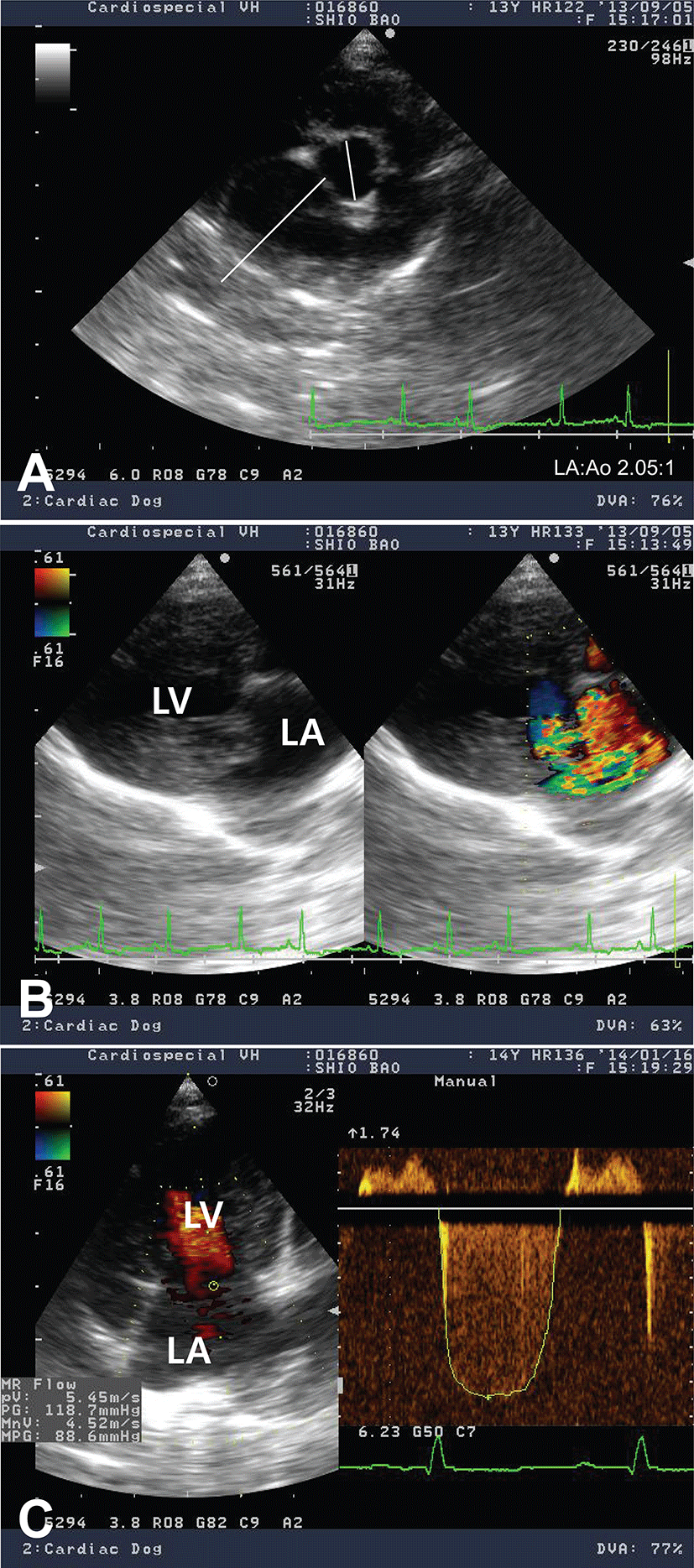
Electrocardiogram showed sinus arrhythmia with left ventricular enlargement pattern (>2.5 mV in R-wave). Thoracic radiography revealed generalized cardiomegaly with marked left atrial dilation, dorsal displacement of trachea, pulmonary infiltration with venous distension, and the compression of main bronchial branches, indicating a typical radiographic sign of DMVD in dogs (Fig. 1). In an echocardiographic study, the left ventricular (LV) cavity size was severely increased and the left atrial (LA) was severely dilated (LA to aorta ratio 2.05:1; Fig. 2A). Color Doppler echocardiography revealed a regurgitant jet from LV to LA by thickened parietal (posterior) and septal (anterior) cusps of mitral valve indicating mitral valve endocardiosis (Fig. 2B). Continuous wave Doppler study also found severe mitral regurgitant jets (5.45 m/s, pressure gradient ~118 mmHg; Fig. 2C). Echocardiographic findings were consistent with an advanced stage of DMVD. Based on diagnostic findings, this case was diagnosed as DMVD with ISACHC IIIa heart failure.
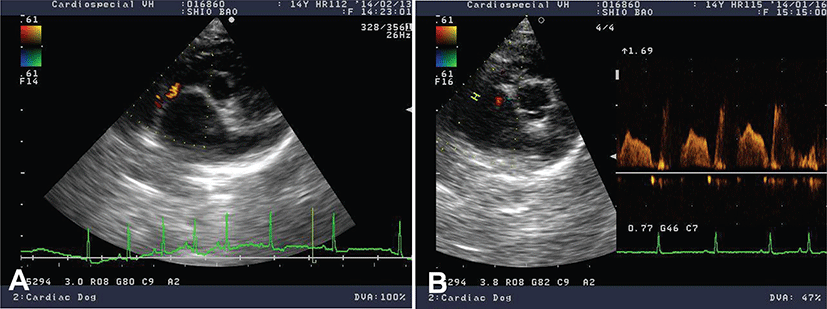
Immediate medical treatment was directed to reduce LA volume overload (furosemide, 0.75 mg/kg, q12 hr, PO; Lasix, Handok, Korea) to inhibit renin-angiotensin-aldosterone system (RAAS; benazepril, 0.5 mg/kg, q24 hr, PO; Benace, Norvatis, Korea) and to improve LV systolic function (pimobendan, 0.25 mg/kg, q12 hr, PO; Vetmedin, Boehringer Ingelheim, Germany). Her clinical condition was stabilized after the administration of cardiac medication. Ten months later, the dog came back to the clinic because of sudden worsening of clinical signs with severe pulmonary edema and several episodes of syncope. The echocardiographic study found pulmonary hypertension (tricuspid regurgitation velocity 3.52 m/s, pressure gradient 49.5 mmHg) on top of DMVD. Medication adjustment including the increase in dosage of furosemide to 1 mg/kg and pimobendan to 0.3 mg/kg as well as the addition of amlodipine of (Norvasc, Pfizer, Korea) 0.1 mg/kg q24 hr was made and the clinical signs were stabilized in 2 weeks. The patient was returned in 4 months for cardiac recheck without obvious worsening of clinical signs. Incidental finding of left-to-right atrial septal defect from the rupture of atrial septum secondary to marked left atrial dilation by DMVD was noted by echocardiography (Fig. 3). To lessen the left atrial volume overload, in addition to adding spironolactone (Aldactone, Pfizer, Korea) 1 mg/kg q12 hr, the frequencies of both furosemide and pimobendan were increased (i.e. from q12 hr to q8 hr). Based on diagnostic findings, this case was re-diagnosed as acquired atrial septal defect (ASD) secondary to rupture of the atrial septum with advanced stage of DMVD. After this medication of medical therapy, the condition of dog was stabilized. Each month, the dog is being monitored. To date, the dog is still survived. No further worsening of clinical signs was noticed in a regular cardiac examination.
Discussion
Congenital ASD is the most common type of ASD and resulted from congenital heart defect in atrial septum causing blood shunt between the left and right atria [10, 11], while acquired ASD is a rare type of ASD and could happen due to any disease condition causing atrial rupture (e.g., bacterial endocarditis and degenerative mitral valve disease; 13-16, 18, 19). Atrial rupture can be occurred by atrial infarction, fatty degeneration of cardiac muscle, trauma, valvular heart disease, cardiac tumor, and aneurysm of the atrium in human [12]. One human respective study found the atrial ruptures were associated with chronic rheumatic mitral valve diseases [12]. The elevation of atrial pressure by mitral valvular regurgitation might be contributed to the rupture of atrium. Several cases of atrial rupture associated with DMVD have been reported in dogs [14-16, 19]. One of the reports has described the rare occasion of atrial septal rupture induced by marked left atrial pressure overload from advanced DMVD [19].
Mitral regurgitation by DMVD is the most common cause for volume overload in LA and pressure overload in pulmonary venous vasculatures [1, 2, 13, 20]. If this condition left untreated, the weakened atrial septal wall (especially at the lesion of fossa ovalis) can be ruptured by LA volume and pressure overload and starts to allow the left to right (L-R) shunt. The L-R shunt will also cause the increase in filling pressure of the right heart (preload) and force the right ventricle to pump out more blood. This constant overloading of the right chambers will thus cause pressure/volume overload in the entire pulmonary vasculature (i.e., pulmonary hypertension). If this pulmonary hypertension is left untreated, it will cause that the pressure in the right chamber become greater than the left chamber, resulting in shunt reversal. In this case, the worsening of pulmonary hypertension might be explained by the L-R shunt from atrial septal rupture.
In this case, the dog initially only had a moderate mitral regurgitation caused by a degenerative valvular disease. However, with time, the increase in regurgitant volume in LA might cause LA pressure overload, which resulted in rupture of atrial septum through weakened fossa ovalis causing the L-R shunting in this dog. Our diagnostic studies clearly revealed the severe mitral regurgitation (~70% of regurgitant fraction), marked dilation of LA (~2.05:1 of LA/Ao ratio), and shunt flow between two atria. The pulmonary hypertension might be due to the L-R shunting flow through ASD as well as a pulmonary venous congestion from advanced stage of a degenerative valvular disease. Although the dog had an evidence of right ventricular pressure overload, the dog only showed clinical signs related to left sided heart failure (e.g. coughing and dyspnea). It may be because the pulmonary hypertension was not severe enough to cause right sided heart failure and/or because early medical intervention had retarded the progression of right sided heart failure in this dog. The role of L-R shunting from ASD might in part be beneficial to reduce the LA volume and pressure overload, however, it could cause the worsening of pulmonary hypertension leading to right sided heart failure later in time. Transcatheter atrial septal defect closure with Amplatzer atrial septal occlude has been documented in dogs [9], we could not attempt because of pre-existing severe pulmonary hypertension in our case.
In conclusion, this case describes a rare case of acquired ASD due to complication of DMVD in a dog. To the authors’ best knowledge, this is the first case report in Asia describing the acquired ASD caused by rupture of atrial septum from the severe LA pressure/volume overload by a degenerative mitral valve disease.