Introduction
Atopic dermatitis (AD) is a representative allergic disease associated with hypersensitivity type 1 reaction. AD has various diagnostic features including eczematous change, xerosis, and pruritus [1]. Pruritus, an intense itching sensation, is one of the most important symptoms of inflammatory skin diseases. The pruritus can be diminished with high-potency topical corticosteroids, but there is some adverse effects such as suppression of the hypothalamic-pituitary-adrenal axis [2].
Conjugated linoleic acid (CLA), known as a dietary fatty acid, was discovered from cooked beef [3]. Two isomers of CLA, trans-10, cis-12 (10t-12c) and cis-9, trans-11 (9c-11t), have preventive effect in tumor development for several carcinogen-induced animal models [4, 5]. Dietary CLA could increase production of IgM, IgG, and IgA from lymph nodes and splenocytes while IgE levels were reduced [6, 7]. In a mouse model of allergic sensitization, 9c-11t CLA reduced total allergen-induced IgE [8]. CLA alleviated birch pollen allergy in human [9] and allergic airway inflammation in mice [8]. CLA reduced systemic inflammatory mediators such as the extracellular matrix metalloproteinases (MMP)-9 and MMP-2 and the cytokines, tumor necrosis factor (TNF)-α, interleukin (IL)-2 and IL-8 in healthy young adults [10]. It was demonstrated that an enriched milk fat inhibits the development of allergic dermatitis in mice [11]. Although anti-pruritic effect of CLA has been reported [12], its specific mechanism has not been identified yet. Pruritus related to AD seems to be linked with serotonin and histamine induced by mast cells [13, 14]. The objective of this study is to determine whether pre-administration of CLA suppresses pruritus in mice, and if so, whether this effect is associated with levels of serum histamine and prostaglandin (PG)E2.
Materials and Methods
A liquid CLA mixture, composed of 36.25% 9c-11t CLA, 36.95% 10t-12c CLA, 1.12% 9c-11c and 1.94% t9-t11 CLA, was kindly donated from Professor Yeong L. Ha (Department of Agricultural Chemistry, Gyeongsang National University, Jinju, Korea). Compound 48/80 (composed of N-methyl-p-methoxyphenethylamine and formaldehyde), carboxymethyl cellulose (CMC) sodium salt, and disodium chromoglycate (DSCG) were purchased from Sigma-Aldrich Co (St. Louis, MO, USA).
Five-week-old male BALB/c mice (SPF grade, 17~19 g) were commercially purchased from Samtako Bio Korea (Osan, Korea) and maintained for one week before the start of the experiments. The mice were housed under standard conditions (temperature of 22 ± 1°C and relative humidity of 55 ± 10%) for 12 hours light/dark cycle. They were fed a laboratory diet and distilled water. Experiments were always done in the afternoon (between 12 p.m. and 1 p.m.). All experiments were carried out at the laboratory animal research center of Chungbuk National University. The procedure was approved by the ethics committee of Chungbuk National University.
CLA was emulsified in 0.5% CMC as a solvent and DSCG as a antipruritic substance was dissolved in saline solution for oral administration. Compound 48/80, one of pruritus induction agents, was dissolved in saline solution for subcutaneous injection and used to induce scratching behavior in BALB/c mice. DSCG at dose of 10 mg/kg and CLA at doses of 200 mg/kg were given orally once on day 1, day 2 and day 3 using a mouse sonde. Untreated and compound 48/80 injected groups were administered with saline solution and 0.5% CMC, respectively, by the same volume as in CLA group (n=7 per group). Compound 48/80, 3 mg/kg, was injected subcutaneously into the back of mice to induce scratching behavior except the untreated control.
The incidences of scratching behavior on the site of injected area after administration of compound 48/80 were counted for 30 minutes. Antipruritic activity (%) was calculated by the following formula: (number of scratches in control group − number of scratches in treated group) × 100 ⁄ (number of scratches in control group).
To examine the serum histamine and PGE2 levels, the mice were anesthetized by anesthetic ether after counting the scratching behavior. Whole blood was obtained by caudal vena cava using syringe. The blood was immediately centrifuged at 130 × g for 7 minutes and the serum was stored at −71°C until measuring the histamine or PGE2.
Serum histamine concentration was assayed using the histamine enzyme immunoassay (EIA) kit (Oxford Biomedical Research Inc., Rochester Hills, MI, USA). The serum was diluted 10 times with phosphate buffered saline (PBS). Standards and the diluted samples (50 μL) were added to corresponding wells on the microplate in duplicate. Fifty μL of histamine-horse raddish proxidase (HRP) conjugate was added to each well. The plate was incubated at room temperature for 45 minutes. The plate was washed five times with 300 μL of diluted wash buffer per well. Fifty μL of tetramethyl benzidine (TMB) substrate was added to each well. The plate was incubated at room temperature for 30 minutes. One normal HCl (50 μL) was added each well to stop the color reaction. The plate was read at 450 nm using an automated microplate reader (Bio Tek Instruments, Inc., Winooski, VT, USA).
PGE2 concentration was assayed using the histamine EIA kit (Oxford Biomedical Research Inc.). The serum (25 μL) samples were added to the corresponding wells on the microplate in duplicate. Diluted PGE2-HRP conjugate (50 μL) was added to each well. The plate was incubated at room temperature for one hour. The plate was washed five times with 300 μL of diluted wash buffer. One hundred and fifty μL of TMB substrate was added to each well. The plate was incubated at room temperature for 30 minutes. One normal HCl (50 μL) was added to each well to stop the color reaction. The plate was read at 450 nm using an automated microplate reader (Bio Tek Instruments, Inc.).
All statistical analyses were performed using GraphPad prism 6 software (GraphPad Software, San Diego, CA, USA). The one-way analysis of variance (ANOVA) followed by Dunnett’s test for each pair for multiple comparisons was used in order to determine the statistical significance of the differences between the control and treatment groups. Comparisons of two groups were made by t-test. P values of less than 0.05 were considered to be statistically significant. All data are expressed as means ± standard errors of the mean (SEM).
RESULTS
To examine the effect of CLA on pruritus induced by compound 48/80, frequency of scratching behavior in compound 48/80 injected mice was observed. As shown in Fig. 1A, the scratching behavior was significantly increased by compound 48/80 injection compared with untreated control (P<0.001). The scratching behavior also showed considerably higher frequency in CLA plus compound 48/80 (P<0.01) and DSCG plus compound 48/80 groups (P<0.001) than that of untreated control group. However, oral administration with CLA significantly suppressed the numbers of scratching behavior induced by compound 48/80 injection (P<0.05). Although the mice treated with DSCG plus compound 48/80 showed the tendency to reduce the scratching behavior compared to compound 48/80 alone, there was no significance between CLA plus compound 48/80 and DSCG plus compound 48/80 groups. In antipruritic activity (Fig. 1B), CLA plus compound 48/80 treated mice showed 48.55% of protection from scratching incidence, while DSCG plus compound 48/80 group indicated 26.8% of protection.
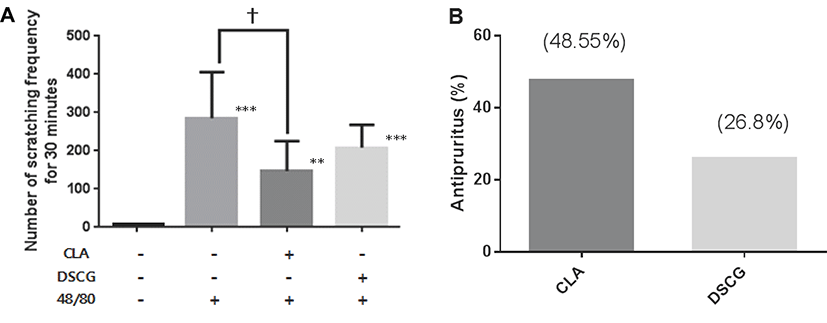
To investigate the effect of CLA on serum histamine concentration, the level of histamine in serum was measured. As shown in Fig. 2, compared with untreated control group, there was a significant increase (P<0.001) in serum histamine concentrations in compound 48/80 alone, CLA plus compound 48/80 or DSCG plus compound 48/80 group. However, the increase in levels of serum histamine concentration by compound 48/80 injection was significantly reduced by oral administration of CLA (P<0.001) or DSCG (P<0.01), respectively. But there was no significance between CLA and DSCG in compound 48/80 groups.
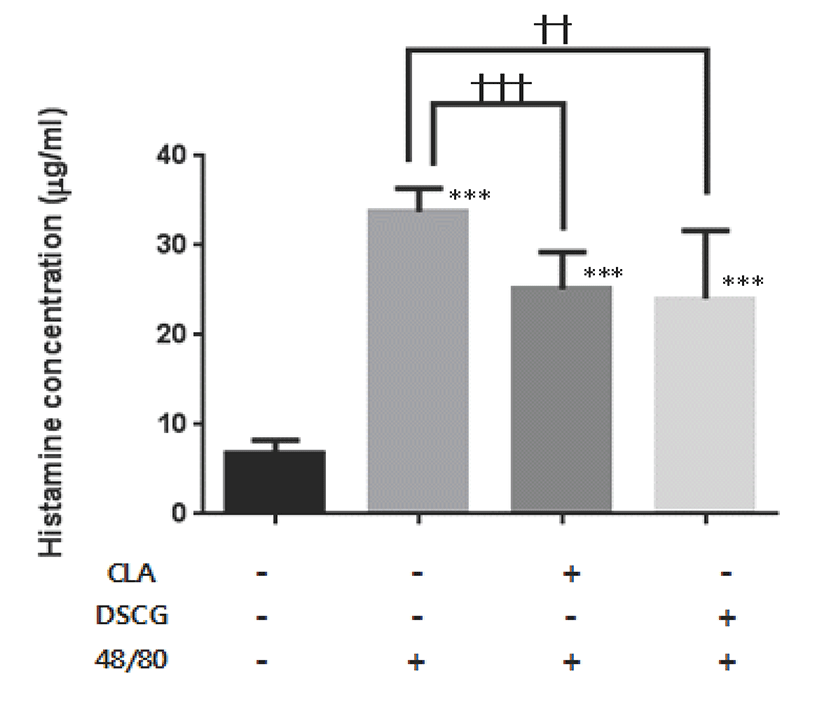
To examine the effect of CLA on serum PGE2 concentration, the level of PGE2 in serum was also measured. Compound 48/80 injection did not increase the level of serum PGE2 compared to untreated mice. The levels of serum PGE2 in administration with CLA (P<0.001) or DSCG (P<0.001) in combination with compound 48/80 injection were lower than that in untreated group. However, the serum PGE2 concentration in compound 48/80 injected mice was significantly decreased by CLA (P<0.01) or DSCG (P<0.01) administration. But there was no significance between CLA and DSCG in compound 48/80 groups. Fig. 3.
Discussion
Scratching is a behavior that results from itching sensation. Scratching response in animals has been used for quantitative analysis of itching without influence of mental conditions [15]. Control of itching and scratching is an important strategy in the therapy of AD and resistant dermatitis [16]. Compound 48/80 as a pruritus induction agent produces an itching sensation and evokes histamine release from mast cells [17, 18]. Here, we used compound 48/80 to examine the effects of CLA on the scratching response in mouse model. Antipruritis activity was estimated by counting of scratching behavior following subcutaneous injection of compound 48/80. In the present study, injection of compound 48/80 to mice skin remarkably induced scratching behavior. In addition, oral administration of CLA reduced compound 48/80-induced scratching behavior. Histamine, arachnoids, neuropeptides, and other chemical mediators have been reported to stimulate nociceptor [19]. Histamine is a major mediator of itching. Injection of histamine to skin induces itching sensation. Histamine receptor antagonism and mastocytes degranulation inhibition are important for suppression of itching [20]. Therefore, we measured histamine as an itching mediator in serum.
Compound 48/80 injection causes mast cell activation and histamine release by up-regulation on H1 and H2 receptors [21]. The data showed that administration of CLA reduced the increase in histamine level induced by compound 48/80 injection. Both H1 and H2 receptors participate in the immediate systemic reactions and H2 receptor-blocking therapeutics are used in alleviating symptoms of anaphylaxis [22]. But the inhibition of compound 48/80-induced scratching behavior occurs mainly via H1 antagonistic activity [23]. Thus, the decrease of histamine level in the present study might be associated with H1 receptor. It was thought that CLA could also suppress serum histamine by way of blocking the H1 receptor. This result implicates that CLA would be useful in allergic pruritus as a preventive medicine. It has been also reported that pre-administration of CLA reduced sneezing [9]. Moreover, anaphylaxis in ddY mice was alleviated by CLA consumption [12].
PGs are products of the cyclooxygenase (COX) pathway in the metabolism of arachidonic acid (AA), and exert a variety of actions mediated by the respective receptors [24]. PGs are notably produced in inflammatory tissues and generally recognized to be potent mediators of pain and inflammation [25, 26]. The present results showed higher level of serum PGE2 in untreated mice than in compound 48/80 injected mice although it is not statistically significant. It has been known that compound 48/80 inhibits phospholipase C activity [27]. PGE2 concentration seems to be declined by compound 48/80 through inhibition of phospholipase C activity. It is possible that blood level of PGE2 is not involved in the skin scratching. The elevation of cutaneous PG production induced by mechanical scratching is involved in the repair of cutaneous damage caused by scratching [28]. This may be associated with cutaneous levels but not blood level of PGs.
However, our results revealed that pre-administration of CLA reduced PGE2 level in serum compared to compound 48/80 injected mice. Dietary CLA repressed the generation of PGE2 in biological mass including serum, spleen and bone [29, 30]. The damage by scratching elevates PGs levels, which may have a physiological role in the repair of the skin damage [28]. It has been reported that the production of PG just after scratching is related with COX-1 rather than COX-2, because COX-1 expression generated only under the rapid condition otherwise COX-2 began to increase after 3 to 24 hours from damages. COX-1 inhibition also enhances scratching behavior in mice with atopic dermatitis [31]. It was, therefore, supposed that CLA prevents compound 48/80-induced skin damage by forestalling COX-1 pathway so that there is no need to repair skin barrier and to produce PGE2. The present findings suggested that CLA can be effective for the prevention of pruritus by decreasing the serum PGE2 level through precluding COX-1.
In conclusion, dietary CLA has an antipruritic effect by down-regulating serum histamine and PGE2 in compound 48/80-induced scratching behavior of mice and it will be useful in allergic pruritus as a preventive medicine.