Introduction
Stress has been associated with infectious diseases, including bacteria, viruses, and parasites [1-3]. For example, cold stress was positively correlated with the incidence and severity of infection with several types of cold viruses [4]. In different strains of mice, repeated restraint stress significantly decreased the clearance of influenza herpesvirusinfection [3]. Until now, mechanisms of disease progression of alphaherpesvirus remain unclear. However, cofactors such as stress are suspected to make a role in the stimulation of its pathogenicity. Canine herpesvirus (CHV) is a member of the alphaherpesvirus subfamily that can cause a severe hemorrhagic disease in neonatal pups as well as mild or subclinical respiratory infections in adult dogs [5, 6]. Since we reported firstly its case in Korea, CHV has been identified in many cases and a very popular distribution is presumed in this country [5-7]. We found that most of its fatal consequences were occurred in neonates during winter season and suggested that cold stress may play a major factor of disease progression and severity of CHV, an alphaherpesvirus [6].
The aims of the present study were to examine the effect of factors on disease progression of CHV, an alphaherpesvirus, in neonatal puppies as a major causative agent of canine reproductive failure.
Materials and Methods
Twelve newborn Beagle puppies (four males, eight females) were used for this experiment. They were delivered from three CHV-seronegative bitches. Eight newborn puppies were challenged intranasally with 104 TCID50 CHV in the same manner as previously described [6] and divided into a cold (10°C) treatment group and a hyperthermal (36°C) group. Four pups were left uninoculated as controls and divided into a cold and a hyperthermal group. We decided the temperatures for two groups by referring to the other report [7]. All studies were performed in accordance with the Guide for Animal Experimentation by Wonkwang University (Approval No. WKU12-39). All efforts were made to minimize pain or discomfort of animals used.
To assess the sickness behaviors in terms of body weight (BW), we weighed puppies daily from day 0 to the day of sacrifice during experiments. The puppies were checked for clinical signs at least three times daily, in the morning, at noon, and in the afternoon. The studied animals were sacrificed at 5 days after initiation. Before sacrifice, urination was induced by palpation stimuli of lower abdomen of studied animals and urine test was performed with reagent strip for urine analysis (Multistix 10 SG, Bayer, USA) according to the manufacturer’s instruction. Thereafter, the blood was drawn from the jugular vein of the studied animals. Blood was used for hematological and chemical analyses. Plasma for these determinations was prepared by centrifugation at 3,000 × g for 10 min at 4°C, after which aliquots were stored at −20°C.
Hematological analysis of the blood samples was performed with an automatic counter (ADVIA 120, Bayer, USA) according to the manufacture’s protocol. Serum biochemical analysis of the gerbils was performed with an automatic analyzer (DRI-CHEM FDC3500, Fuji, Japan) according to the manufacturer’s instructions.
Nasal swabs were taken daily until the completion of the study. Complete necropsies were done immediately after euthanasia. Tissues specimens were collected from brain, trigerminal ganglia, palatine tonsils, spinal cord, heart, lungs, trachea, nasal mucosa, liver, spleen, lymph nodes, kidneys, urinary bladder, ovaries, uterus, adrenals, thyroid, salivary glands, stomach, small intestine, large intestine, pancreas, skeletal muscle, and skin of infected and noninfected animals. For virus isolation and polymerase chain reaction (PCR) analysis, some of the tissues of trigerminal ganglia, brain, lung, and kidney were collected freshly and submitted to processing for each analysis. For histopathological observation, collected tissues were fixed in 10% buffered formalin. After fixation, tissue was embedded in paraffin in an usual manner, sectioned (4 μm), and stained with hematoxylin and eosin (H&E).
Virus was isolated from trigerminal ganglia, brain, lung, and kidney of each animal as previously described [5-6, 8]. Tissues were homogenized and resuspended in Minimum Essential Medium (MEM) and suspensions were filtered through a membrane with a pore size of 0.45 μm. Madin Darby canine kidney (MDCK) cells were grown in 48-well microtiter plates and inoculated in duplicate with 30 μL of each previously filtered swab sample. Inoculated cell cultures were incubated for 24 hr at 37°C and 5% CO2 and then the media were replaced to minimize possible toxic effects of the inoculum to the cells. Plates were incubated for additional 5 days and checked daily for cytopathic effect (CPE). Supernatants of wells with visible CPE were harvested and assayed by PCR to confirm the presence of CHV DNA.
DNA was extracted from nasal swabs, brain, lung and kidney of each animal. Tissues were homogenized and resuspended in phosphate buffered saline (PBS). Also, nasal swabs were suspended in PBS, and suspensions were submitted to DNA extraction as previously described [5]. Approximately 500 ng of DNA was used as PCR template. A nested PCR assay for detection of the CHV thymidine kinase gene (TK) sequence was used as described elsewhere [9], which predicted PCR products of 508 base pairs (bp) for the first round and 336 bp for the nested round of amplification, respectively.
The sequences of the first-round primers were forward F1 5’-CTGGCGTATCATCCTAGAAACA-3’ and reverse R1 5’-CCCACTCTTTATACCAATCGT-3’. The sequences of the second-round primers were forward F2 5’-TGCGTGGTGAATTAAGCCTA3’ and reverse R2 5’-CGCGATCTTTTTTTTAAGCG-3’. For the first-round of amplification, 500 ng of template DNA was added to the PCR mixture consisting of 50 mM KCl, 10 mM Tris–HCl, 1.5 mM MgCl2, 0.4 mM each dNTP, 100 pmol of each primer (Gibco BRL, USA), and 1.25 units of Taq polymerase (Gibco BRL, USA). After an initial denaturation step of 5 min at 95°C, samples were amplified for 35 cycles of 1 min denaturation at 95°C, 1 min annealing at 55°C, and 1 min extension at 72°C, followed by a final 5 min extension step at 72°C. First-round PCR product (1 μL) was used as template for the nested-round of amplification. Analysis of amplified DNA was performed in agarose gels.
Results
In order to confirm the hypothesis that puppies subjected to cold stress at the time of infection with CHV might be at greater risk than ones of a hyperthermal treatment group, we first assessed abnormal clinical signs associated with administration of CHV in neonatal puppies. All in a cold stress group resulted in a nearly 100% occurrence rate of abnormal signs among the puppies inoculated with CHV. The symptoms were body weight loss, nasal discharge, salivation, painful crying, petechial red foci, anorexia and dyspnea on the skin. They showed those symptoms from 2 days post inoculation (d.p.i.) and its severity had been increased by passage time. On 5 d.p.i, those animals fell into very fatal status and were submitted to euthanasia. However, in hyperthermal treated puppies inoculated with CHV, only one showed clinical symptoms and others showed no abnormal signs. No clinical signs were observed in both a cold treatment and a hyperthermal group of non-infected puppies.
In the puppies of cold stress treatment group with CHV-infection, hematological analysis indicated severe decrease of platelet and white blood cell (WBC) (Table 1). In those animals, biochemical analysis showed the increase of alanine transaminase (ALT), asparate transaminase (AST), blood urea nitrogen (BUN), total bilirubin, and creatinin (Table 2) and urine analysis revealed high grades of leukocyte, urobilinogen, protein, ketone, bilirubin, and glucose (Table 3). However, in hyperthermal treated puppies inoculated with CHV, only one showed mild changes in hematological and urine analysis and others did not show any abnormal changes (Table 1, 2, 3). No analytical changes were observed in both cold treatment and hyperthermal group of non-infected puppies (Table 1, 2, 3).
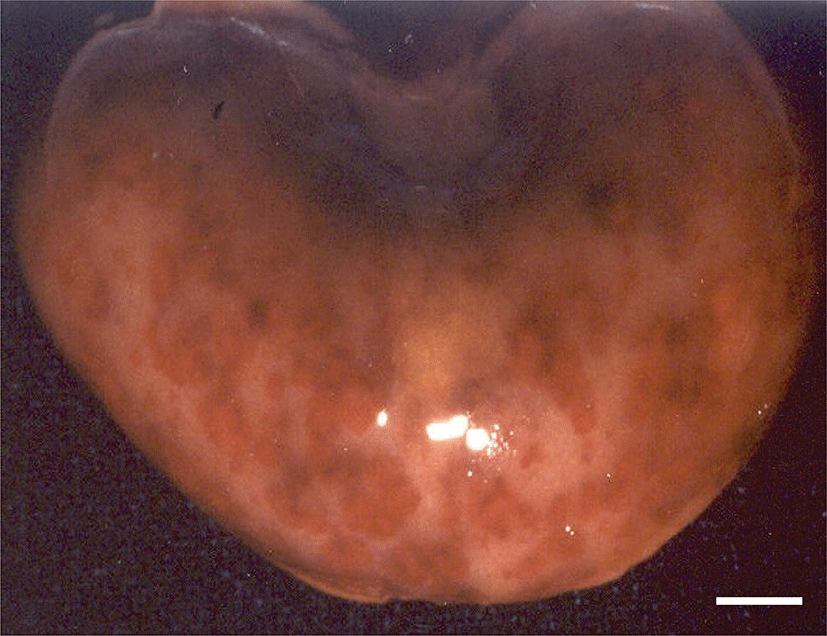
Pathological changes were observed in the organs of the puppies with clinical symptoms. Grossly, lots of petechial red foci were scattered throughout the lungs, kidneys, livers and intestines of all CHV-infected puppies exposed to cold stress. The kidneys contained multifocal hemorrhages apparent from the capsular surface, measured 2~3 mm in diameter (Fig. 1). The lungs were diffusely mottled red to dark red, slightly firm, and noncollapsing. The livers from both pups had multiple and pinpointed red foci apparent from the capsular surface on cut sections. Their lymph nodes and spleens were enlarged. In the group of CHV-infected puppies exposed to hyperthermal treatment, one of the animals had slight to moderate petechial foci on the lungs. In CHV-uninoculated animals, no abnormalities were observed.
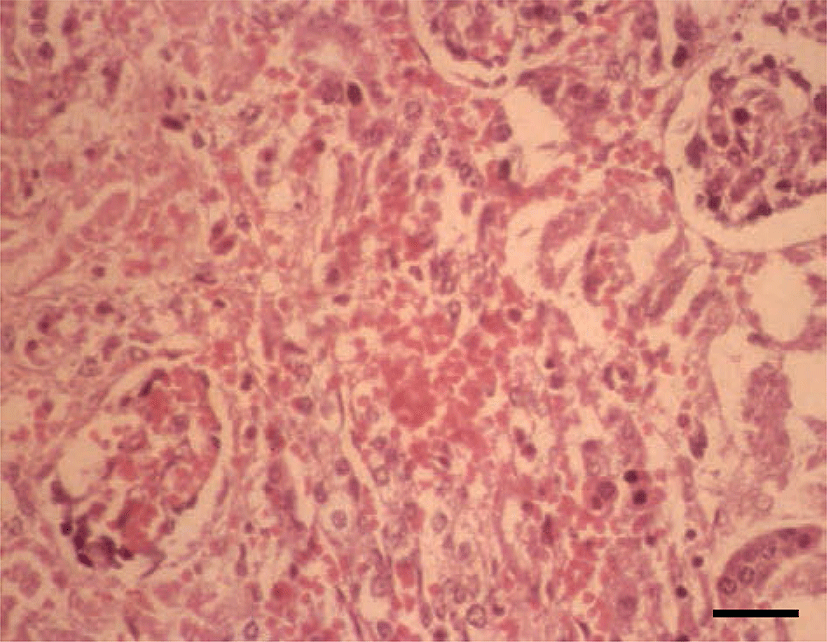
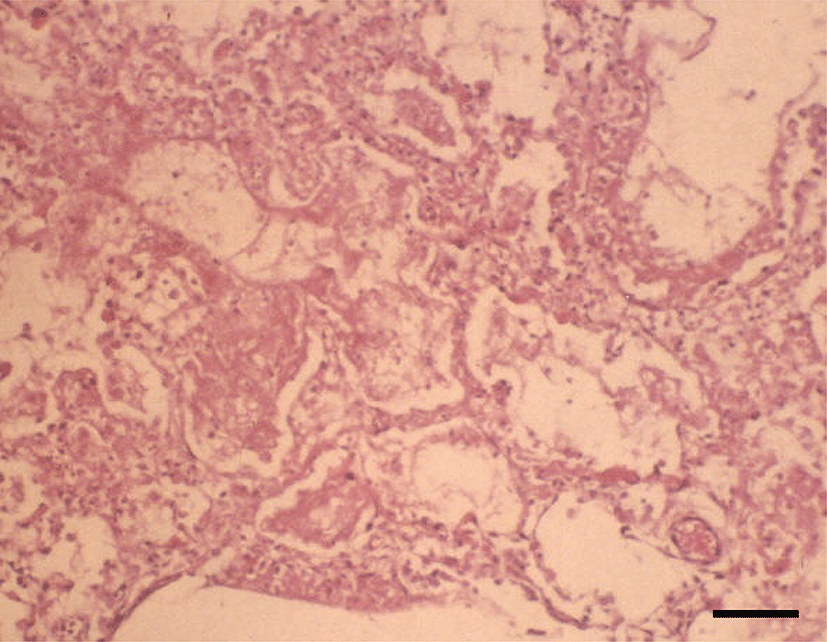
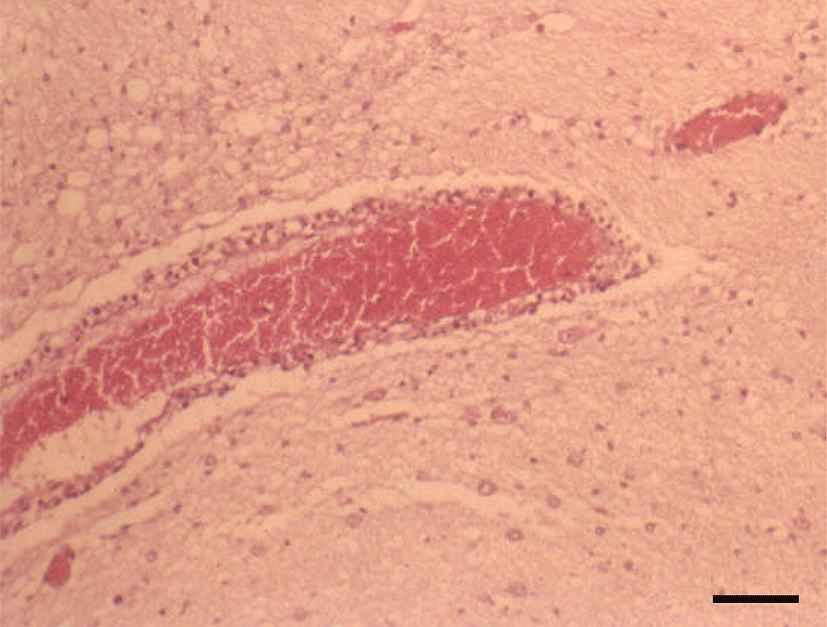
Histopathologically, in the kidney, renal tubular epithelial cells were swollen with hypereosinophilic cytoplasm and absent of pyknotic nuclei, and necrotic cell debris filled tubular lumina. Glomerular tufts contained karyorrhectic debris and increased eosinophilic matrix within the mesangium. Endothelial cells lining blood vessels were disrupted and hemorrhage extended into the necrotic foci (Fig. 2). In the lung, disrupted alveolar septa were replaced by karyorrhectic debris and fibrin strands. Fibrin, hemorrhage, and foamy alveolar macrophages filled alveoli in affected areas (Fig. 3). Histopathological findings of the infected brains showed the presence of nonsuppurative meningoencephalitis, characterized by microgliosis, degeneration and necrosis of neurons and glial cells, and perivascular cuffing of lymphocytes (Fig. 4). However, in the group of CHV-infected puppies exposed to hyperthermal treatment, only one of the animals had a slight to moderate multifocal necrosis on the lungs and liver. In CHV-uninoculated animals, no abnormalities were observed.
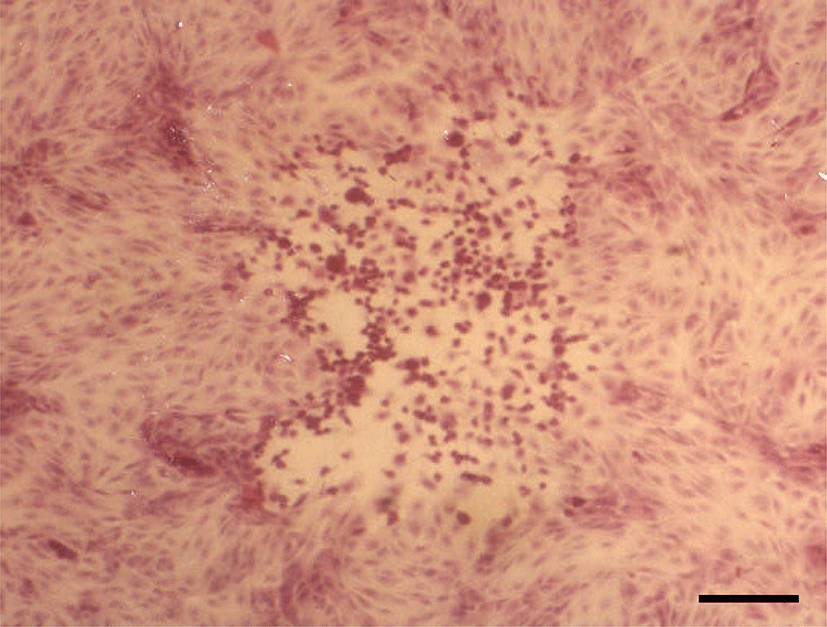
Virus was isolated from the tissues in trigerminal ganglia, brain, lung, and kidney of the CHV-infected puppies with clinical symptoms (Table 2). The cytopathic effect (CPE) was observed on CHV-isolated cultures. Infected cells were rounded and detached from the glass surfaces, leaving clear plaques surrounded by necrotic cells (Fig. 5). The virus was identified as CHV by type specific PCR. CHV was not isolated from the tissues of non-inoculated animals.
The nucleic acids of CHV were detected from the nasal swabs of CHV-infected puppies exposed to cold stress from 3 days after CHV inoculation. However, in the CHV-infected puppies exposed to hyperthermal treatment and CHV-uninoculated animals, no viral DNA was detected from their nasal swabs. CHV-specific nucleic acids were detected from the tissues in trigerminal ganglia, brain, lung, and kidney of the CHV-infected puppies with clinical symptoms by type specific PCR (Fig. 6). However, in the CHV-infected puppies exposed to hyperthermal treatment, viral DNAs were detected only in trigerminal ganglia except on pup with clinical symptoms. CHV was not isolated from the tissues of non-inoculated animals.

Discussion
There is a growing evidence that psychological and physical stress have a significant impact on health and disease, including neurodegenerative diseases such as Parkinson’s disease [10], autoimmune diseases such as systemic lupus erythematosus [11], and infectious diseases [2, 12]. Stress can modulate multiple aspects of immune responses to antigens/invading pathogens, including both innate and adaptive immunity, and both cell-mediated and humoral immunity [13-17]. Often, stress increases susceptibility to infectious diseases, which has been demonstrated both in humans and animals [4, 18, 19]. Physical or psychological stress can modulate immune responses in normal subjects. Here we assessed relationships among cold stress and infection with CHV in neonatal puppies. This study was designed to characterize an animal cold stress/infection model to aid investigation of the underlying mechanisms involved in stress suppression of host resistance to a virus infection and assess the involvement of disease progression and clinical consequences during its infection. In the challenged cold treatment group, all of the pups showed CHV-related disease within 5 days; most of them showed typical clinical signs and macroscopic lesions, and CHV infection confirmed by the isolation of the virus. However, in the challenged thermoneutral group, only one of the pups died of CHV-induced disease at 8 days post inoculation. None of the puppies in the uninoculated group died although they were exposed to cold stress. These findings indicate that cold stress can cause rapid disease progression of CHV, an alphaherpesvirus. The biological changes displayed by individuals in reaction to acute stressors, may be important factors for explaining variability in stress-induced susceptibility to infectious diseases [20-22]. The underlying assumption here is that the stress-induced responses of the immune system are thought to modulate host resistance to infectious agents. Knowing the propensity of individuals to respond in a specific way in the laboratory should increase our ability to predict if they will respond to natural stressors with increased or decreased susceptibility. Commonly studied response to stressor is an increase in the levels of the steroid hormone cortisol that is released by the adrenal cortex. Cortisol can suppress the inflammatory immune response and the release of more cortisol under stress could be associated with greater susceptibility to infectious agents [23]. In this work, cold stress induces rapid disease progression in neonatal pups infected with CHV. These results might be induced by the decreased immune function similarly with cortisol-mechanism. In this study, we sought to expand our understanding of the association between cold stress and infectious illness. Previously, we found that most of its fatal consequences were occurred in neonates during winter season [6] and suggested that cold stress may play a major factor of disease progression and severity of CHV, an alphaherpesvirus. In this study, we confirmed the negative effect of cold stress against the disease progression of alphaherpesvirus by animal experiment.
We had studied factors on disease progression of CHV, an alphaherpesvirus, in neonatal puppies as a major causative agent of canine reproductive failure. In summary, this study demonstrates that cold stress can substantially suppress host resistance to infection and enhance sickness behaviors.