ARTICLE
Unilateral noncystic renal dysplasia in a Sprague Dawley rat
Yong-Hoon Lee1, Duyeol Kim1, Sun Hee Park1, Mi Ju Lee1, Myoung Jun Kim1, Ho-Song Jang1, Jin Seok Kang2,*, Jongkoo Kang1,3,*
*Corresponding author: Jin Seok Kang, Department of Biomedical Laboratory Science, Namseoul University, Cheonan 331-707, Korea Tel: +82-41-580-2721, Fax: +82-41-580-2932, E-mail:
kang@nsu.ac.kr; Jongkoo Kang, College of Veterinary Medicine, Chungbuk National University, Cheongju 361-763, Korea, Tel: +82-43-261-2607, Fax: +82-43-271-3246, E-mail:
jkkang@cbu.ac.kr
© Research Institute of Veterinary Medicine, Chungbuk National University. All rights reserved. This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: Apr 23, 2014; Revised: Jun 12, 2014; Accepted: Jun 12, 2014
Abstract
Renal dysplasia is a developmental disorder of the renal parenchyma involving anomalous differentiation. It is characterized by persistent metanephric ducts surrounded by primitive mesenchyme, fetal or immature glomeruli, fetal or immature tubules, interstitial fibrosis, and dysontogenic metaplasia involving tissues such as cartilage. Renal dysplasia has been rarely reported in rats. Here, we observed a small left kidney in a rat used in a short-term repeat toxicity study. The rat showed no clinical signs throughout the study. All parameters, including those reflecting kidney functions, were normal upon hematological examination and urinalysis. Grossly, the kidney was small (5 × 8 mm) and its surface appeared normal. Histological examination revealed that the cortex and medulla were poorly demarcated and contained immature/atrophic glomeruli, immature renal tubules, and mesenchymal cells. The cortex contained immature glomeruli, atrophic glomeruli with cystic dilatation of Bowmans capsular space, and some atypical tubules. Primitive metanephric tubules in the medulla were larger in diameter than normal collecting ducts, lined by a tall columnar epithelium with pale cytoplasm and basal nucleus, and surrounded by loose mesenchymal cells. Occasional tubules contained pale eosinophilic homogenous material in the lumen. Thus, this was diagnosed as a case of renal dysplasia on the basis of histologic features and is the first reported case of renal dysplasia in Sprague Dawley rats.
Keywords: renal dysplasia; kidney; Sprague Dawley rat; pathology; case report
Introduction
Renal dysplasia is a developmental disorder of the renal parenchyma involving anomalous differentiation and disruption in the normal development of the collecting duct system, that is the so-called renal branching morphogenesis in which transcription factors, growth factors, and cell signal peptides are involved [1]. In humans, renal dysplasia is well documented as one of the main causes of childhood end-stage renal failure [2]. It has also been reported in animals including dogs, cats, cattle, horses, pigs and sheep [1, 3-8]. In rodents, renal dysplasia has been reported in the Wistar rat and Syrian hamster [9, 10]. However, there have been no reports of renal dysplasia in the Sprague Dawley rat. To the best of our knowledge, this is the first reported case of renal dysplasia in the Sprague Dawley rat.
Case report
A ten-week-old female Sprague Dawley rat with a small left kidney was discovered accidentally during necropsy of control animals in a short-term repeat toxicity study (four weeks). The rat was kept in a polycarbonate cage and received commercial rodent diet (Harlan, the Netherlands) and purified autoclaved water ad libitum. Environmental conditions were controlled to provide a temperature of 18~26°C, a relative humidity of 30~70%, and a 12:12 hr light: dark cycle. All housing procedures and experiments were performed in accordance with Biotoxtech Co., Ltd. Institutional Animal Care and Use Committee Guidelines (Accepted No. 110510).
The animal showed no clinical signs throughout the study, and hematological examination and urinalysis did not reveal evidence of disturbed kidney function. At necropsy, the left kidney was found to be small (5 × 8 mm) with a normal surface (Fig. 1). On the cut surface of the kidney, the cortico-medullary ratio was markedly decreased. The right kidney was normal in size with no gross lesions, and no other organs had obvious macroscopic lesions.
Fig. 1.
Gross findings in the left kidney of a Sprague Dawley rat. The kidney is small in size (5 × 8 mm). Bar=5 mm.
Download Original Figure
The kidneys were resected and fixed in 10% neutral buffered formalin, processed routinely, embedded in paraffin, sectioned in 3 μm thick slices, and stained with hematoxylin and eosin (H&E). In addition, selected sections were stained with Alcian blue (pH 2.5) and Masson’s trichrome stain to differentiate fibrosis from mesenchymal tissue.
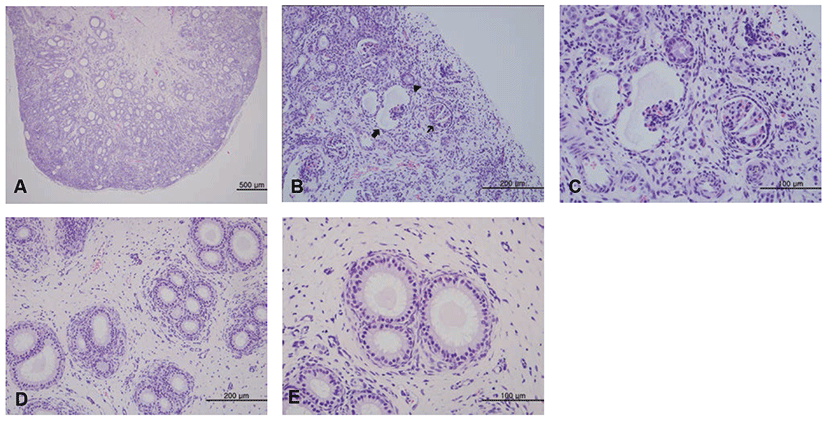
Histological examination revealed that the cortex and medulla were poorly demarcated and contained immature/atrophic glomeruli, immature renal tubules, and mesenchymal cells (Fig. 2A). The cortex contained immature glomeruli, atrophic glomeruli with cystic dilatation of Bowman’s capsular space, and some atypical tubules (Figs. 2B and 2C). Primitive metanephric tubules in the medulla were larger in diameter than normal collecting ducts, lined by tall columnar epithelium with pale cytoplasm and a basal nucleus, and surrounded by loose mesenchymal cells (Fig. 2D). The mesenchymal cells displayed mild staining with Alcian blue but no staining with Masson’s trichrome (data not shown). Occasional tubules contained pale eosinophilic homogenous material in the lumen (Fig. 2E). On the other hand the right kidney appeared normal on histological examination.
Fig. 2.
Histological findings of renal dysplasia (H&E staining). (A) The cortex and medulla are poorly demarcated. Bar=500 μm. (B) Immature glomeruli (thin arrow), atrophic glomeruli with cystic dilatation of Bowman’s capsular space (thick arrow), and atypical tubules (arrow head) are seen in the cortex. Bar=200 μm. (C) High magnification of Fig. 2B. Bar=100 μm. (D) The medulla contains primitive tubules lined by tall columnar epithelium with pale cytoplasms and a basal nucleus, and surrounded by loose mesenchymal cells. Bar=200 μm. (E) Occasional primitive tubules contain eosinophilic material in the lumen. Bar=100 μm.
Download Original Figure
Discussion
Renal dysplasia is generally defined as an abnormality that results from disorganized development of renal parenchyma associated with altered differentiation of specific cellular elements of renal tissue [1, 11]. This implies that the ureteric bud and the metanephric blastema interacted abnormally during development [12]. Characteristics of renal dysplasia include persistent metanephric ducts surrounded by primitive mesenchyme, fetal or immature glomeruli, fetal or immature tubules, interstitial fibrosis, and dysontogenic metaplasia involving tissues such as cartilage [13]. This case was characterized mainly by immature and fetal glomeruli and primitive metanephric tubules surrounded by loose mesenchymal cells. Thus, the present case was diagnosed as renal dysplasia on the basis of histological findings. The presence of fetal glomeruli as well as atypical tubule epithelium and fetal tubules indicates that the metanephric blastema developed but differentiated incompletely, and the presence of primitive metanephric ducts surrounded by mesenchymal cells and dysontogenic metaplasia signifies that the ureteric bud and the metanephric blastema failed to interact completely [5]. Renal dysplasia should be differentiated from hypoplasia and neoplasia: hypoplsia is characterized by decreased nephron number and kidney size but no abnormal tissue, and neoplasia is invasive and usually includes only one disordered cell type [11].
A previous report of renal dysplasia case in a Wistar rat describes primitive glomerular/tubular structures, tubules resembling collecting ducts and mesenchyme [10]. These findings are in accord with the histological characteristics observed in the present study. Interestingly, among the criteria for renal dysplasia, fibrosis and dysontogenic metaplasia have not been observed in rat cases. In contrast, fibrosis and dysontogenic metaplasia have often been observed in other animals including dog, cat, calf, and hamster [3, 9, 13-16]. Although these histological differences in renal dysplasia are probably attributed to species differences or experimental conditions, more cases are required to elucidate the cause.
In humans, kidney malformations with subsequent neoplasia are associated to chromosomal abnormalities and are often accompanied by other malformations [10]. In domestic animals, cases of familial renal disease in several dog breeds often have dysplastic features [1]. Renal dysplasia reported in Japanese black cattle is due to deletion of the paracellin-1 gene of chromosome 1 [17]. Furthermore, renal dysplasia in puppies is caused by neonatal infection with canine herpes virus [18], in kittens by fetal infection with feline panleukopenia virus [19], and in calves by fetal infection with bovine viral diarrhea virus [20], and renal dysplasia in pig has been attributed to vitamin A deficiency [1]. However, the exact cause in rats has not been determined.
While renal dysplasia in domestic animals such as dog has been well reported in the literature because of its clinical significance, renal dysplasia in rats has rarely been documented. Therefore, this report provides an example of renal developmental background lesions in rats.
Acknowledgements
This study was supported by Biotoxtech Co., Ltd. We are grateful to all the researchers who contributed to this study.
REFERENCES
Maxie MG, Newman SJ. In: Jubb KVF, Kennedy PC, Palmer N, editors. The urinary system. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals. 2007; 5th edPhiladelphiaSaunders p. 259-330.

Woolf AS, Price KL, Scambler PJ, Winyard PJ. Evolving concepts in human renal dysplasia. J Am Soc Nephrol. 2004; 15 p. 998-1007.

Ohara K, Kobayashi Y, Tsuchiya N, Furuoka H, Matsui T. Renal dysplasia in a Shih Tzu dog in Japan. J Vet Med Sci. 2001; 63 p. 1127-1130.

Ohba Y, Kitagawa H, Okura Y, Kitoh K, Sasaki Y. Clinical features of renal tubular dysplasia, a new hereditary disease in Japanese Black cattle. Vet Rec. 2001; 149 p. 115-118.

Picut CA, Lewis RM. Microscopic features of canine renal dysplasia. Vet Pathol. 1987; 24 p. 156-163.

Plummer PJ. Congenital renal dysplasia in a 7-month-old quarter horse colt. Vet Clin North Am Equine Pract. 2006; 22 p. e63-e69.

Ronen N, van Amstel SR, Nesbit JW, van Rensburg IB. Renal dysplasia in two adult horses: clinical and pathological aspects. Vet Rec. 1993; 132 p. 269-270.

Sasaki Y, Kitagawa H, Kitoh K, Okura Y, Suzuki K, Mizukoshi M, Ohba Y, Masegi T. Pathological changes of renal tubular dysplasia in Japanese black cattle. Vet Rec. 2002; 150 p. 628-632.

Ikeyama S, Nishibe T, Furukawa S, Goryo M, Okada K. Unilateral Renal Dysplasia in a Syrian Hamster. J Toxicol Pathol. 2001; 14 p. 309-312.

Ruehl-Fehlert CI, Deschl U, Kayser M, Hartmann E. Bilateral noncystic renal dysplasia in a Wistar-rat. Exp Toxicol Pathol. 2003; 54 p. 293-299.

Frazier KS, Seely JC, Hard GC, Betton G, Burnett R, Nakatsuji S, Nishikawa A, Durchfeld-Meyer B, Bube A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol Pathol. 2012; 40 p. 14S-86S.

Morita T, Michimae Y, Sawada M, Uemura T, Araki Y, Haruna A, Shimada A. Renal dysplasia with unilateral renal agenesis in a dog. J Comp Pathol. 2005; 133 p. 64-67.

Kerlin RL, Van Winkle TJ. Renal dysplasia in golden retrievers. Vet Pathol. 1995; 32 p. 327-329.

Aresu L, Zanatta R, Pregel P, Caliari D, Tursi M, Valenza F, Tarducci A. Bilateral juvenile renal dysplasia in a Norwegian Forest Cat. J Feline Med Surg. 2009; 11 p. 326-329.

Bruder MC, Shoieb AM, Shirai N, Boucher GG, Brodie TA. Renal dysplasia in Beagle dogs: four cases. Toxicol Pathol. 2010; 38 p. 1051-1057.

Testoni S, Mazzariol S, Drogemuller C, Piffer C, Aresu L, Gentile A. Renal dysplasia in grey Alpine breed cattle unrelated to CLDN16 mutations. Vet Rec. 2012; 170 p. 22.

Ohba Y, Kitagawa H, Kitoh K, Sasaki Y, Takami M, Shinkai Y, Kunieda T. A deletion of the paracellin-1 gene is responsible for renal tubular dysplasia in cattle. Genomics. 2000; 68 p. 229-236.

Albert DM, Lahav M, Carmichael LE, Percy DH. Canine herpes-induced retinal dysplasia and associated ocular anomalies. Invest Ophthalmol. 1976; 15 p. 267-278.

Greco DS. Congenital and inherited renal disease of small animals. Vet Clin North Am Small Anim Pract. 2001; 31 p. 393-399.

Anderson ML. Infectious causes of bovine abortion during mid- to late-gestation. Theriogenology. 2007; 68 p. 474-486.