Introduction
Leiomyoma arising primarily from the vagina of a bitch is a common tumor, but the mass from the ovary is very rare [1]. To date, few cases have been reported in veterinary medicine [2-4]. To our knowledge, only one case was reported especially in a dog [2]. Since leiomyoma is very low prevalence and asymptomatic, its features and characteristics have seldom been described. Histologically, leiomyoma consists of mostly smooth muscle cells with no mitotic figures [5-9]. It features spindle cells with elongated, blunt-ended nuclei and eosinophilic cytoplasm that form interlacing fascicles [6-9]. Futhermore, it also needs to be differentiated with other ovarian tumors, thecoma, fibrothecoma and leiomyosarcoma [1, 6]. Differential diagnosis of leiomyoma very similar with fibroma and thecoma has similar morphology with leiomyoma except vacuoles in the cells in hematoxylin and eosin staining [1, 6]. Therefore, Immunohistochemical staining is usually required to diagnose a mass. In immunopathological findings, leiomyoma is positive immunoreactive for α-smooth muscle actin and desmin, negative for vimentin and S-100 [6, 8, 10]. Meanwhile, leiomyosarcoma is positive immunoreactive for vimentin and desmin, less positive for α-smooth muscle actin and S-100 [10]. Fibroma is positive for vimentin and negative for α-smooth muscle actin [11]. For these reasons, we determined the gross, histopathological and immunohistochemical findings to differentiate them.
Case report
The abnormal ovary of a 10-year old female Yorkshire Terrier was presented from a local animal hospital for diagnosis. The history was as follows. The dog had an abdominal mass from a pelvic ultrasonography was found and the mass was on the right side of the uterus. A laparotomy revealed a large multi-nodulated ovarian mass. The mass was found in the right ovary and was clearly separated from the uterus. The left ovary and uterus were normal and an oophorectomy was performed. Tissue was fixed immediately in 10% neutral buffered formalin and submitted for microscopic examination at the Department of Pathology, Kyungpook National University. All samples were processed routinely and embedded in paraffin. The tissue was cut into 4 μm sections and stained with hematoxylin and eosin (HE). For immunopathological studies, the primary antibodies used were α-smooth muscle actin (α-SMA) (Sigma Co, St. Louis, MO, USA), vimentin (Dako, Carpenteria, CA, USA), S-100 (Dako, Carpenteria, CA, USA) and desmin (Dako, Carpenteria, CA, USA). The antigen-antibody complex was visualized by the avidin-biotin complex immunoperoxidase systems using a commercial kit (Vectastain Elite ABC kit; Vector Laboratories Inc., Burlingame, CA, USA) with 3,3-diaminobenzidine. Immunohistochemical stained sections were counterstained with Mayer’s hematoxylin.
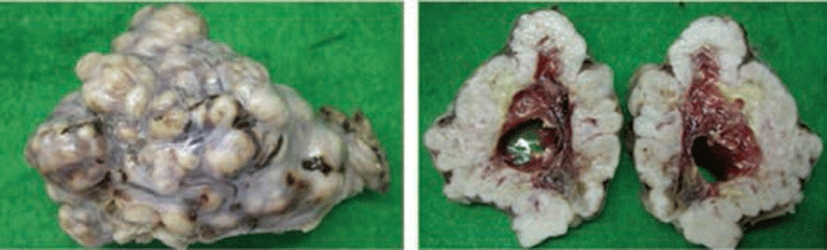
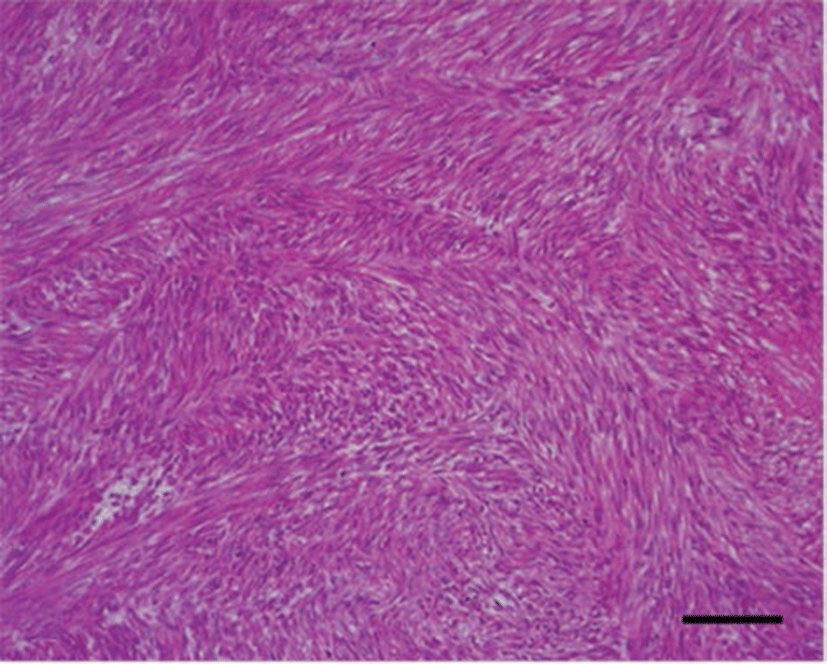
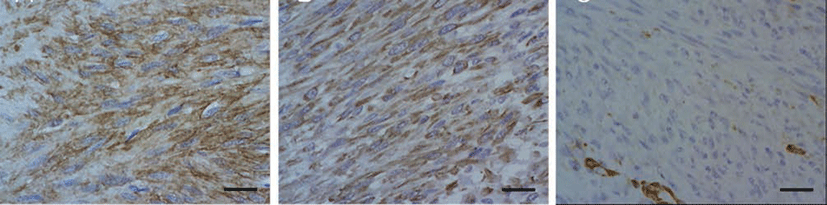
Grossly, the firm mass measured 12 × 7 × 6 cm with a weight of 173 g. It was multi-nodulated and covered with white thin capsules that were suspected to be tunica albuginea (Fig. 1A). The cut surfaces of the mass showed multiple firm whitish nodules in the cortex (1.5~2.0 cm diameter) and the medulla consisted of thin, bloody, loose connective tissue (Fig. 1B). Microscopy showed that the cortex of the mass consisted mostly of spindle cells with uniform nuclei with blunt ends and eosinophilic cytoplasm. The cells formed interlacing fascicles running various angles so that some bundles were visible in the longitudinal section and others in the cross-section (Fig. 2). These cells lacked criteria of malignancy such as namely cellular pleomorphism, moderate to marked anisokaryosis, moderate to high mitotic figure, invasiveness, hemorrhages or tumor necrosis. In the immunopathological findings, the tumor cells showed strong and diffuse positive staining for α-SMA (Fig. 3A) and desmin (Fig. 3B), whereas, negative staining for vimentin and S-100, except for the endothelial cells for vimentin (Fig. 3C).
Discussion
In dogs, leiomyoma have been commonly detected in the vagina and sometimes gastrointestinal tract and uterine [1, 10, 12]. However, there are few documented cases of leiomyoma in the ovary of a dog [2]. Therefore, this case is valuable information to veterinary medicine and veterinary practitioners likely to encounter cases of ovarian leiomyoma.
In humans, primary ovarian leiomyomas are less uncommon than in dogs and mostly encountered in women aged between 20 and 65 years [5]. Most of that are found incidentally in routine examinations, autopsy, surgery, mass screening, since the tumors are usually asymptomatic, rarely become large [6, 7]. The sizes were usually less than 3 cm in diameter but the largest reported tumor measured 36 × 32 × 25 cm with a weight of 11.65 kg [7]. They are enough to present pelvic pain and increased abdominal girth [7]. However, in our case, ovarian leiomyoma was not large enough to induce pain or increase abdominal girth. Occasionally, the gross features of ovarian leiomyoma reported were just enlarged or cystic structures [6, 13]. The cut surfaces of them showed a whorled pattern or cystic change with a honeycomb appearance [6, 13]. Other changes included hyalinization, fibrosis, calcification, epitheliod change and symplastic change [14]. However, in our case, these features were not presented.
Distinguishing ovarian leiomyoma other ovarian tumor such as fibroma, thecoma and fibrothecoma was required in the present case. Morphologically, fibroma is composed of fibroblasts which are, in general, more slender than leiomyoma cells. The thecoma cells are spindle shaped cells and has elongated nuclei but vacuoles in the cells [1, 13]. The cells of the present mass, however, were spindle shape with elongated, blunt-ended nuclei and eosinophilic cytoplasm. In addition, immunohistochemical stianing is useful in establishing the differential diagnosis. The smooth muscle origin of the tumor was generally detectable, staining with antibodies such as desmin, α-SMA and vimentin [5, 6, 10, 12]. The diffuse, strong positive staining for desmin and α-SMA are characteristic of leiomyoma, and staining for vimentin may help in more accurately defining tumors of questionable malignancy [10, 13]. A further aid in distinguishing smooth muscle cell tumors with peripheral nerve sheath tumor [PNST] is the use of antibodies to the neural antigen S-100 [15]. Our results showed positive staining for desmin and α-SMA, while vimentin and S-100 antigen were not identified, thus supporting a diagnosis of leiomyoma. For more accuracy, magnetic resonance imaging [MRI] or staining with masson’s trichrome and sudan black also can help diagnose [1, 5].
In this report, we described the gross, histopathological and immunohistochemical features about a case of an ovarian mass in a dog, suggesting that these findings in the ovary were compatible with those of leiomyoma. Rare ovarian leiomyoma needs to be considered in the differential diagnosis of other tumors that are apparently composed of spindle-shaped cells. Furthermore, this case also provides valuable information to veterinary medicine and veterinary practitioners what are likely to encounter cases of ovarian leiomyoma in dogs.