Introduction
Clostridium difficile infection is associated with broad-spectrum antibiotic therapy that alters the normal gastrointestinal flora and is the most common cause of infectious diarrhea in hospital patient [1, 2]. So it is a significant cause of morbidity and mortality among elderly hospitalized patients. Pathogenic strains of C. difficile produce two protein exotoxins, toxin A and toxin B, which cause colonic mucosal injury and inflammation [1, 3]. Infection may be asymptomatic, cause mild diarrhea, or result in severe pseudomembranous colitis (PMC). PMC is characterized by the presence of yellow or white plaques on the mucosal surface that are composed of inflammatory exudates. Risk factors for PMC include antimicrobial therapy, older age (>65 years), antineoplastic chemotherapy, length of hospital stay, and procedures or medications that alter the intestinal motility or flora [4, 5]. Antimicrobial exposure is the greatest risk factor for patients. The antibiotics most frequently implicated in predisposition to PMC include fluoroquinolones, clindamycin and broad-spectrum penicillins and cephalosporins [6]. However, any antibiotic can predispose to colonization by C. difficile, including metronidazole and vancomycin, which are the major antibiotics used to treat C. difficile infection [3]. The use of broad-spectrum antimicrobials, use of multiple antibiotic agents, and increased duration of antibiotic therapy all contribute to the incidence of PMC.
Most antituberculosis agents have few effects on intestinal flora, so PMC associated with antituberculosis therapy is very rare [7-12]. Although few cases of PMC associated with antituberculosis therapy were seen most frequently in patients with pulmonary tuberculosis, but there is no article that with tuberculous meningitis which is to be treated for long periods of time.
We therefore document a case of PMC associated with antituberculosis therapy in a patient with tuberculous meningitis.
Case report
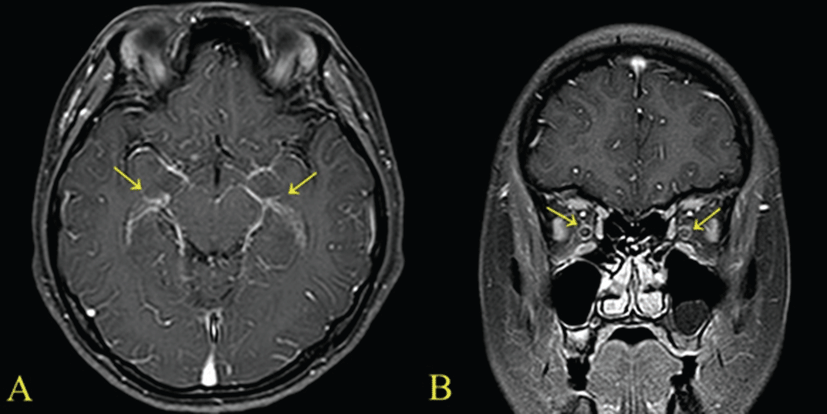
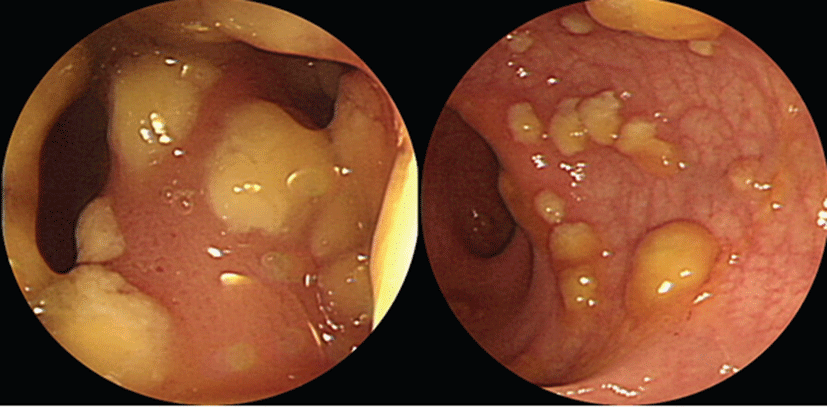
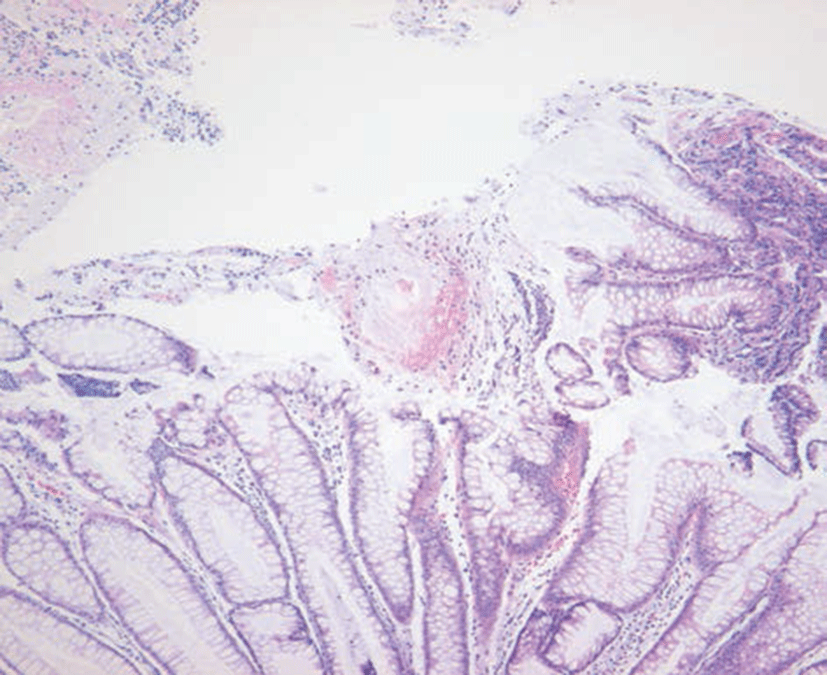
A 29-year-old female patient was admitted due to headache and diplopia that had lasted for 2 weeks. She was previously healthy and had not recently received antimicrobial therapy. She complained of the insidious onset of malaise, lassitude, protracted headache, and visual disturbance including blurred vision and diplopia. On physical examination, her vital signs were within normal limits and there was no distinct sign of meningeal irritation. For differential diagnosis of eye diseases, careful fundoscopic examination was conducted and revealed no abnormality bilaterally. So lumber puncture was the cornerstone of diagnosis. Examination of cerebrospinal fluid (CSF) revealed a high leukocyte count (103/uL), with a predominance of lymphocytes (94%), elevated protein content (64.9 mg/dL), and within a low-normal glucose concentration (66 mg/dL). Magnetic resonance imaging (MRI) of the brain showed markedly increased leptomeningeal & optic nerve enhancement (Fig. 1). She was diagnosed with tuberculous meningitis by CSF findings of low-normal glucose concentration, elevated protein, and lymphocytic pleocytosis and neurologic examination including brain imaging study. But examination of the CSF by acid-fast and culture was negative. Intravenous dexamethasone (0.4 mg/kg per day) is administered for faster resolusion of CSF abnormalities and elevated CSF pressure. She was also treated with standard antituberculosis agents (HERZ regimen), including isoniazid (300 mg/day), rifampicin (600 mg/day), pyrazinamide (1,500 mg/day), and ethambutol (1200 mg/day). Eleven days after initiation of antitubercular treatment, she complained of fever, chilling sense, sudden widespread skin eruption and itching sense. Her serum white blood cell (WBC) count was 12,200/mm3. Aspartate aminotransferase and alanine aminotransferase were markedly elevated at 57 IU/L and 185 IU/L respectively. Considered adverse drug reactions, antituberculosis agents were stopped. And conservative treatments for fever and skin lesion were performed including antipyretics and antihistamines. One week later, her symptoms were relieved significantly. Her WBC count and liver function also had returned to normal. To identify the cause of adverse effects, antituberculosis agent was sequential reintroduced one at a time by daily interval. She was started on escalating doses of individual antituberculosis drugs one after another according to the World Health Organization guidelines. She tolerated isoniazid, rifampicin and ethambutol till the full doses. So we assumed that pyrazinamide was the cause of adverse effects. Two days after reintroduction of antitubercular treatment, she presented with frequent mucoid, jelly like diarrhea and lower abdominal pain. On physical examination, her bowel movements were very frequent and abdomen was soft and flat. Tenderness was noticed in the left lower quadrant of the abdomen without rebound tenderness. From stool WBC was not found, but serum white blood cell count was 13,110/mm3 and high sensitive C-reactive protein was elevated at 2.87 mg/dL. The initial stool test for C. difficile cytotoxin was negative. Although oral anti-diarrhea agent was administered, symptoms lasted for 10 days. She was experiencing diarrhea 5 to 6 times per day. Sigmoidoscopy revealed that multiple yellowish plaque lesions up to 2 cm in diameter were scattered over from the rectum to the sigmoid colon (Fig. 2). And mucosal biopsy from the sigmoid colon showed small collections of neutrophils between crypts at the summit of the mucosa with tufts of damaged surface epithelium (Fig. 3). Follow up stool test for C. difficile cytotoxin was positive. So she was diagnosed with PMC. Because risks of drug interactions between antitubercular agents and metronidazole were expected, she was treated with oral vancomycin 250 mg four times per day with intravenous fluid and electrolytes. Her symptoms resolved slowly within 2 weeks of oral vancomycin treatment, during which she completely recovered from diarrhea, tenesmus, and abdominal pain. We continued antitubercular agents, replacing pyrazinamide with the second line drug levofloxacin 500 mg once per day. She experienced no recurrence of diarrhea by the end of treatment, and her general condition recovered including skin lesions and visual disturbances.
Discussion
Because of the variety of side effects and drug interactions, the use of antituberculous drugs should be carefully considered in its selection and monitor [13]. Especially, extrapulmonary tuberculosis such as tuberculous meningitis might need more caution because of its relatively long-term treatment [14]. The main side effects include neurotoxisity, hepatotoxcity, dermatologic reaction, and hematologic reaction, but PMC induced by antituberculous drugs is rarely reported [13]. Rare cases of antitubercular agents-associated PMC have been reported since the 1980s [15-17]. Most C. difficile strains are susceptible to antitubercular regimen including rifampicin, but resistance can develop rarely.
Well-known side effects related with gastrointestinal track are abdominal pain, nausea, and vomiting [13]. The mucoid, jelly-like diarrhea upto 10 or 15 times daily with lower abdominal pain and cramping, and low-grade fever are the distinctive symptoms of patients with PMC, and multiple elevated yellow plaques are observed in endoscopical study, other than edematous and erosive mucosa presented in common enteritis with diarrhea [18, 19]. These findings contribute to differential diagnosis of PMC from the drug side effects. PMC is commonly localized to the rectum and sigmoid colon. Thus, sigmoidoscopy is usually sufficient to detect typical findings of PMC such as the pseudomembrane. However, there are reports of rectal or rectosigmoid sparing PMC occuring in as many as 23% to 69% of patients [20, 21]. Therefore, total colonoscopy should be performed to confirm the diagnosis of PMC, if sigmoidoscopy is inconclusive.
The mechanism of alteration of normal flora in gastrointestinal track and what kind of drugs including antibiotics might cause such effect are not well established, but the consequence that antibiotics disrupt normal colonic flora, providing a niche for C.difficile to multiply and elaborate toxins [22]. And also, which drug of the standard HERZ regimen might cause PMC is hard to identify, because there is no study of effects of antituberculous drugs on normal flora. But among antitubercular agents, rifampicin has been implicated as a cause of PMC. Because it has antibacterial activity on a broad spectrum of bacteria whereas isoniazid, pyrazinamide, and ethambutol do not have antibacterial activity to disturb the normal intestinal flora. Besides the broad spectrum of antibacterial activity, frequent isolation of rifampin-resistant toxigenic C. difficile also implies that rifampin could be the causative agent [23, 24].
In presenting case, after initiation of antitubercular treatment, our patient complained of fever, chilling sense, sudden widespread skin eruption and itching sense. We estimated that pyrazinamide was the cause of adverse effect. But rarely skin rashes and photosensitivity are reported during the treatment of pyrazinamide [25]. The common side effects due to pyrazinamide are hyperuricemia (gout), hepatotoxicity, nausea, vomiting, flushing, dysuria, arthralgia and sideroblastic anemia. The best method to ascertain whether side effects are induced by pyrazinamide consists of retrial with a single regimen of pyrazinamide and observation of recurrence. However, we did not retry pyrazinamide because our patient tolerated isoniazid, rifampicin and ethambutol till the full doses and was in poor general condition due to her prolonged illness. We achieved a successful result after replacing pyrazinamide with levofloxacin, which suggests that pyrazinamide was responsible for skin lesions and hepatotoxicity.
In most patients with PMC, diarrhea occurs after 5 to 10 days of antibiotic administration [26]. But there is no article that how long it takes to occur PMC after the initiation of antitubercular agent administration. Our patient developed diarrhea 3 weeks after initiating antituberculosis therapy. A relatively long period is not unusual in general antibiotics associated PMC.
Once PMC is diagnosed, it is usually treated with oral therapy with metronidazole 250 mg, 4 times per day for 10 days, is the recommended first-line therapy for PMC [27]. During antibiotic therapy, restoration of beneficial intestinal flora with probiotics may suppress the abnormal overgrowth of C. difficile and thus ameliorate the course of the disease. A regimen of vancomycin, 125 to 500 mg, 4 times per day for 10 days, should be limited to patients who cannot tolerate or have not responded to metronidazole, or administered in the context of metronidazole-resistent organisms such as enterococci [27]. In presenting case, because risks of drug interactions between antitubercular agents and metronidazole were expected, we treated with oral vancomycin.
When a patient who has not received antimicrobial therapy complains of abdominal pain or diarrhea while taking antitubercular drugs, clinicians must keep in mind and should be evaluated for the possibility of antitubercular agent-associated PMC.