Introduction
Canine lymphoproliferative disorders are relatively rare in dogs, but typically include peripheral lymphocytosis and diverse subtypes of acute and chronic lymphocytic leukemia [1, 2, 3]. In acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), and lymphoma with circulating neoplastic cells (stage 5 lymphoma), lymphocytosis is a characteristic feature [1]. However, differentiation of these three disorders is often unclear.
Categorization of lymphoid leukemia of bone marrow origin as ALL or CLL is important with regard to diagnosis, therapy, and prognosis. ALL is characteristically a rapidly progressive disorder associated with proliferation of morphologically malignant, immature lymphoblasts in the peripheral blood and bone marrow [4]. Acute leukemia can also affect lymph nodes and spleen, so it can be difficult to distinguish stage V lymphoma and leukemia [1, 5].
In contrast, CLL is characterized by the neoplastic clonal proliferation of mature, small lymphocytes rather than lymphoblasts [2, 4, 6]. This disorder may also manifest with lymphadenopathy and splenomegaly [7, 8], making the differentiation between stage V lymphoma and CLL unclear. Unlike ALL, CLL generally has a protracted clinical course and is usually initially responsive to chemotherapy.
In this study, we tentatively diagnosed ALL and CLL by use of bone marrow biopsy, and administered chlorambucil to a CLL patient.
Case report
A 6-year-old castrated male Australian cattle dog (case 1), weighing 26.1 kg, presented to the Gyeongsang National University Animal Medical Center with a history of anorexia, lethargy, and dyspnea of one month’s duration. An 8-year-old castrated male Shih-Tzu dog (case 2), weighing 6.5 kg, presented with a history of weight loss and abdominal distension of two weeks’ duration.
On physical examination of case 1, enlargement of superficial lymph nodes was not identified. Initial hemogram results revealed severe anemia, thrombocytopenia, and marked leukocytosis with immature lymphocytes predominating (Fig. 1A). On a peripheral blood smear, immature lymphoid cells, featuring decreased nuclear chromatin condensation and nuclear pleomorphism, were present. Splenomegaly was revealed by radiography in the lateral view of the abdomen. On ultrasonography, we found some reactive lymph nodes in the abdomen. Bone marrow aspiration was performed on the right humerus. The sample revealed mononuclear lymphoid cells of various sizes, with more lymphoblast cells than mature small- to medium-sized lymphocytes (Fig. 1B). We tentatively diagnosed ALL on the basis of the laboratory results and the clinical course. The dog was treated with analgesics, but there was no response. Euthanasia was administered at the client’s request 19 days after ALL was diagnosed.
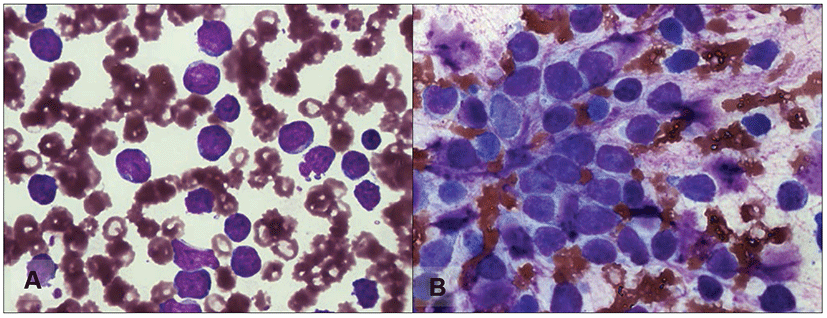
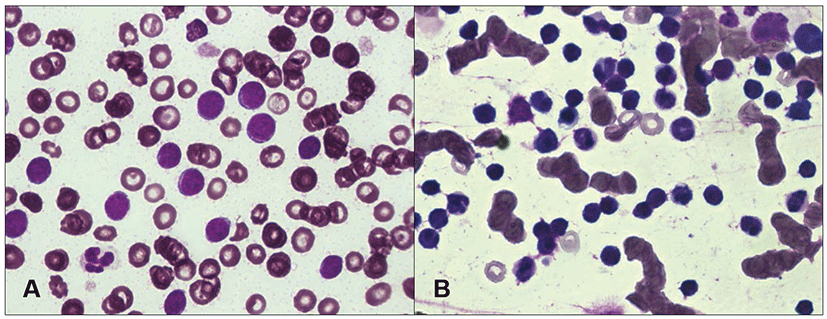
Physical examination of case 2 revealed depression, lethargy, and pale mucous membranes. Superficial lymph node swelling was not noticed in case 2. Initial hemogram results revealed marked leukocytosis with small lymphocytes predominating. Severe anemia and thrombocytopenia were also observed. On examination of a peripheral blood smear, the lymphoid cells were uniform and small in size and had a round nucleus with abundant chromatin (Fig. 2A). Severe splenomegaly and honeycomb sign of the spleen were identified from radiography and ultrasonography. Biopsy samples of the bone marrow revealed hypercellularity and a large number of mononuclear cells similar in shape to the cells observed in the peripheral blood (Fig. 2B). Based on the history of illness, hematological results, and morphological characteristics observed in the bone marrow smear, we tentatively diagnosed CLL and did not perform immunophenotypic analysis. Initially, we treated the dog with vincristine (0.6 mg/m2 body surface area, intravenous) and prednisolone (1 mg/kg body weight, twice a day, oral). The dog’s clinical signs gradually improved, but hematological findings worsened one week later. Treatment was changed to administration of chlorambucil (20 mg/m2 body surface area, once every two weeks, oral) and prednisolone (50 mg/kg body weight, once a day during the first week, then reduced to 20 mg/kg body weight, once every other day, oral). After administration of chlorambucil, the clinical signs waxed and waned. Hematological abnormalities, including leukocytosis with lymphocytosis, gradually decreased, although anemia and thrombocytopenia remained. The patient’s general clinical condition gradually worsened with the development of complications, including superficial corneal ulceration, depression, and severe anemia. To improve the clinical status, we administered eye drops containing N-acetylcysteine, hyaluronic acid, and ofloxacin, and also performed a blood transfusion. The dog died of respiratory failure 89 days after CLL was diagnosed.
Discussion
The diagnosis of canine lymphoid leukemia was established by the clinical course, hematological results, morphological features of peripheral blood and bone marrow smears, and by eliminating other diseases that lead to lymphocytosis. Through examination of the bone marrow smear, we determined the tentative diagnosis based on the morphologic characteristics of the lymphoid cell. An alternative diagnostic approach to identify diverse subtypes of lymphoid leukemia is to perform immunophenotypic analysis by flow cytometry in the same manner as in human medicine [9, 10]. The advantage of using flow cytometry for classifying lymphoproliferative disorders is that this method is more objective than cytology or histology, and may be easier to standardize [1]. In a recent study [11], it was found that clonal rearrangement using polymerase chain reaction (PCR) to amplify the variable regions of the immunoglobulin gene and T-cell receptor gene is a very sensitive assay to detect lymphoid malignancy.
Using chlorambucil and prednisolone is the traditional treatment of canine CLL as well as human CLL [4, 7, 12, 13]. Chlorambucil is a nitrogen mustard alkylating agent which acts as a cytoreductive chemotherapy agent and can be given orally. Thus, it is commonly adopted as a relatively mild drug with fewer side effects than other alkylating agents [12]. In some reports [12], it has been suggested that the alternative choice of melphalan for canine CLL may reduce the risk of clinical infections, but this remains speculative.
A limitation of this report is the lack of immunophenotypic analysis as commonly done in human leukemia patients. In a retrospective study [1], immunophenotyping of neoplastic canine lymphocytosis was found to offer important prognostic information. Several prognostic factors, such as lymphadenopathy, splenomegaly and/or hepatomegaly, anemia, thrombocytopenia, marked lymphocytosis, and rapid lymphocyte doubling time, are associated with poor prognosis [14]. In another study, expression of CD34 as a marker of acute leukemia predicted poor prognosis, and the median survival time was 16 days [13]. Immunophenotyping may help to differentiate acute leukemia and stage V lymphoma, because CD34+ cells did not stain with any of the other surface markers used in that laboratory, although further studies are needed [1]. In previous immunophenotypic analyses in dogs, there have been many cases of T cell CLL that express CD3 and CD8, whereas some cases of CLL derive from B cells [11]. Median overall survival time was 480 days for B cell CLL and 930 days for T cell CLL [14].
Considering the diverse aspects of canine lymphoproliferative disorders, not only bone marrow biopsy but also immunophenotypic analysis may be performed in veterinary medicine as in human medicine, and is helpful to improve the diagnostic specificity and to predict prognosis of canine lymphoid leukemic patients. Because of the similarities with human leukemia, canine leukemia provides a valuable model for researching human leukemia.