INTRODUCTION
Meningoencephalomyelitis of unknown etiology (MUE) is a non-infectious inflammatory neurologic disease in dogs. MUE has been reported to occur in approximately 5%–25% of dogs [1]. MUE is classified as granulomatous meningoencephalomyelitis (GME), necrotizing meningoencephalitis, necrotizing leukoencephalitis, or eosinophilic meningoencephalitis, by histopathologic examination [2].
The prognosis of MUE is guaranteed to be fair and is related to the response to immunosuppressive treatment [3]. It has been reported that determining prognosis based on the treatment effect for recovery in dogs with MUE is challenging because of the difficulty in making definitive diagnoses, disease heterogeneity, treatment variability, and low sample size [4]. Although several prognostic factors of MUE have been investigated, including signalments, clinical signs, neuroanatomical localization, and diagnostic findings such as those from magnetic resonance imaging (MRI), cerebrospinal fluid (CSF) analysis, and blood analysis, obvious prognostic factors have not been identified [2, 5-10].
The exact pathophysiology of MUE is currently unknown; however, autoimmune and genetic pathogeneses are suspected to be the major pathophysiologies of MUE [2]. Therefore, immunosuppressive therapy has been the mainstay of treatment for MUE. Several treatment protocols using different immunomodulatory drugs have resulted in different survival times [3, 4, 6, 7, 11-13]. This study aimed to investigate the prognostic factors in dogs with MUE and to identify the treatment outcomes of multi-drug therapy.
MATERIALS AND METHODS
The medical records of dogs with MUE that visited the Chungbuk National University Veterinary Teaching Hospital between July 2013 and July 2017 were retrospectively reviewed. Diagnosis of MUE was based on the following criteria according to a previous report: 1) age > 6 months; 2) presence of single, multiple, or diffuse intracranial lesions on MRI; 3) presence of pleocytosis (total nucleated cell count [TNCC] > 5 nucleated cells/μL and a red blood cell count < 4,000 cells/μL) with > 50% mononuclear cells in the CSF; and 4) absence of infectious diseases [14]. Signalments; seizure activity; findings from physical and neurological examination, complete blood cell count (CBC), serum biochemistry, and MRI; treatments; and outcomes were used for analysis.
Treatment outcome and prognostic factor analyses were conducted by comparing survival times. The survival time was defined as the duration between the day of MUE diagnosis and the day of death or euthanasia. Successful long-term outcomes were defined as survival times of at least 100 days. For prognostic evaluation, dogs were divided into two groups: those surviving for < 100 days (group A) and those surviving for > 100 days (group B).
The presence or absence of seizures, results of the CSF analysis, lesion volume, neuroanatomic localization (location of lesion), MRI features of the lesion, and signalments including sex, body weight, and age at diagnosis were analyzed to investigate prognostic factors.
The presence of seizures was identified through history taking. Total protein (TP) and TNCC in the CSF were estimated in the CSF analysis. The CSF analysis findings were considered abnormal if the white blood cell count was > 5 cells/mL and TP was > 25 mg/dL [15]. MRI lesion features were determined by the presence of four abnormal MRI characteristics (midline shift, cerebellar herniation, effacement of sulci, and contrast enhancement), and lesion volume was estimated by multiplying the sum of the lesion area in each slice on the transverse T2-fluid-attenuated inversion recovery (FLAIR) MRI images by the thickness of the slice. Neuroanatomic localization was defined as the lesion location on the transverse FLAIR image and classified according to location, including presence only in the forebrain, presence in the brainstem, or multifocal location.
Dogs with MUE were divided into four groups, and each group was treated with prednisolone (PDS) in combination with one of the following immunomodulatory drugs: group 1, cyclosporine; group 2, cyclosporine / cytosine arabinoside (CA) / leflunomide / mycophenolate mofetil (MMF); group 3, CA; and group 4, leflunomide / MMF. Dogs were included in groups 2 and 3 when there were two or more CA treatments at 3-week intervals. In groups 2 and 4, if the treatment response was poor or side effects of the drug were observed, the treatments were replaced.
The PDS treatment was initiated with a dose of 1.5 mg/kg q12h per oral (PO) for 3 weeks and tapered as follows: 1.0 mg/kg q12h PO for 6 weeks, 0.5 mg/kg q12h PO for 3 weeks, 0.5 mg/kg q24h PO for 3 weeks, and 0.5 mg/kg q48h PO indefinitely. If the PDS-related side effects were severe, they were tapered quickly [16].
CA treatment was initiated with a dose of 50 mg/m2 administered subcutaneously (SC) every 3 weeks for 4 cycles. One cycle refers to four SC injections of CA administered 12 hr apart. The treatment interval was adjusted based on the clinical response and the CSF analysis and MRI examination were occasionally repeated. This was extended at intervals of 4, 5, and 6 weeks, if the patient remained in clinical remission. CBC was performed to monitor bone marrow suppression 10–14 days after each CA treatment [14].
Cyclosporine treatment was initiated at a dose of 6–10 mg/kg q24h PO and tapered from 3 to 5 mg/kg q24h PO or from 6–10 mg/kg q48h PO after 4 weeks. The dosage was adjusted to obtain a target blood cyclosporine level of 200–400 ng/mL [17].
Leflunomide treatment was maintained at a dose of 1.5–4 mg/kg q24h PO and its range in the blood was maintained at 20–40 μg/mL [18].
MMF treatment was initiated at a dose of 10–20 mg/kg q12h PO for 4–8 weeks and tapered with a maintenance dose of 5–10 mg/kg q24h PO. If gastrointestinal signs, which are known side effects, occurred or were suspected, treatment was initiated with a lower dose [19]. In case of epilepsy, dogs were treated with anticonvulsants such as phenobarbital, zonisamide, or potassium bromide. Antibiotics and liver protectants have been used to prevent the side effects of immunosuppressive drugs.
Fisher’s exact test and the Kruskal–Wallis test were used to identify differences in signalments (sex, age at diagnosis, and body weight), seizure activity, neuroanatomical localization, the CSF findings (TP, TNCC), and lesion volume in the four treatment groups.
In a survival study, the effect of postulated dependent variables and therapeutic drugs on measured outcomes was assessed using Kaplan–Meier product limit survival analysis and Kruskal–Wallis test. The median survival was calculated for each independent variable. The Cox proportional hazards regression model was used to assess the correlation between survival and lesion volume. For all statistical analyses, values of p<0.05 were considered significant. All calculations were performed using SAS Version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
A total of 23 toy-breed dogs were included in this study. The breeds of dogs included were Maltese (n = 13), Yorkshire Terrier (n = 3), Miniature Poodle (n = 2), Shih Tzu (n = 2), Japanese chin (n = 1), and crossbreed (n = 1). Overall, 13 dogs were female (56.5%), of which 4 were neutered, and 10 were male (43.4%), of which 6 were neutered. Total mean body weight was 3.28 (range, 1.22–6.76) kg and total mean age at diagnosis was 7.07 (range, 0.75–19) years. The number of dogs included in each group according to successful long-term outcomes was as follows: group A, n = 10; group B, n = 13. The number of dogs included in each group according to treatment was as follows: group 1, n = 6; group 2, n = 7; group 3, n = 4; and group 4, n = 7. CSF analysis was performed in only 20 dogs because of the risk involved in CSF tapping.
The overall median survival time after diagnosis was 56 (range, 1–926) days, the median survival time of group A was 41 (range, 1–98) days, and that of group B was 326 (range, 171–926) days. A total of eight dogs were euthanized, six in group A and two in group B. There were no significant differences between groups A and B in sex, body weight, and age at diagnosis (Table 1). Additionally, there was no significant difference in the occurrence of seizures between groups A (69.2%) and B (70%). Further, there was no significant difference between these groups in TP and TNCC in the CSF analysis and location of the lesion at initial diagnosis. The median number of abnormal MRI features in group A (median = 2) was significantly more than that in group B (median = 0.52; p=0.02). However, there were no significant group differences in the numbers of individual features of midline shift, cerebellar herniation, effacement of sulci, and contrast enhancement.
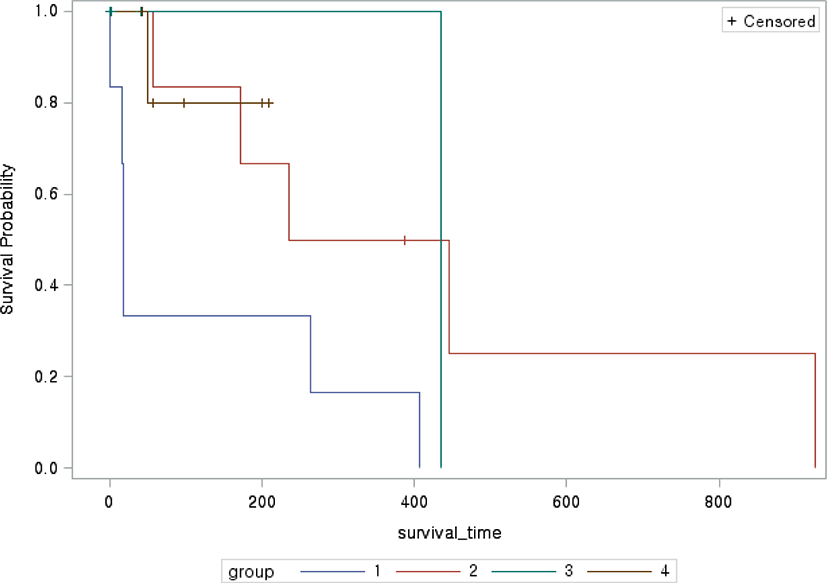
The median survival time in each treatment group ranged from 18 (range, 1–407) days in group 1, 236 (range, 41–926) days in group 2, 21.5 (range, 1–435) days in group 3, and 77 (range, 43–209) days in group 4 (Fig. 1). Group 2, which was treated with the most diverse drugs, tended to have a longer survival time than the other groups. When the survival times of the four groups were compared, the survival curve of group 1 was significantly different from that of group 2 (p=0.04). The Kaplan–Meier survival curves of group 1 tended to differ from those of groups 3 (p=0.052) and 4 (p=0.65). There was no significant difference in signalments, presence of seizure, TP in the CSF analysis, lesion location, or number of abnormal MRI features per dog (Table 2). However, the TP in the CSF analysis (p=0.03) and lesion volume (p=0.02) were significantly different among the four treatment groups.
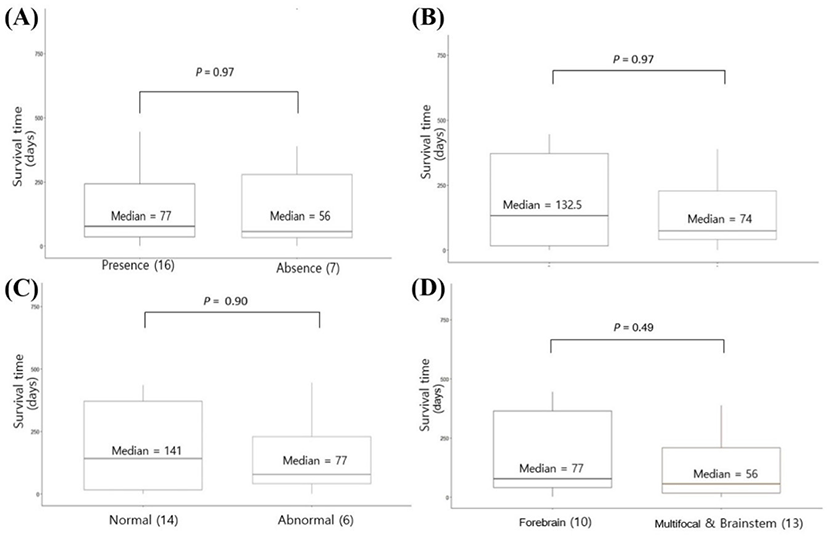
In the survival study, the median survival time was 77 and 56 days for dogs with seizures and without seizures, respectively, and no significant differences according to the presence of seizures were identified (Fig. 2A). There was no significant difference between dogs with (median survival time, 74 days) and without TP abnormalities in the CSF analysis (median survival time, 132.5 days; Fig. 2B). The median survival time was 77 and 141 days for dogs with and without TNCC abnormality in the CSF analysis; however, this difference was not statistically significant (Fig. 2C). The median survival time was 77 and 56 days for dogs with forebrain lesions and multifocal or brainstem lesions, respectively, with no significant difference between the two (Fig. 2D). There was no significant correlation between lesion volume and survival time from initial diagnosis to death (correlation coefficient ρ = –0.04; p=0.70).
DISCUSSION
In the present study, survival time was not affected by sex, body weight, age at diagnosis, the presence of seizures, TP and TNCC in CSF analysis at initial diagnosis, or lesion location. Among the MR images examined, lesion volume was not a reliable prognostic factor. Although individual abnormal features were not significant associated with mortality, presence of abnormal MRI features was significantly associated with it. As a result of these treatments, CA-based multidrug therapy might be effective in dogs with MUE, and replacement with various immunosuppressive drugs might be considered in MUE with drug resistance and adverse effects.
A previous study on prognostic factors showed that young age was associated with a poor prognosis [8]. However, other studies have suggested that signalments may not be a reliable prognostic factor [5, 9]. As in previous studies, the results of the present study suggest that signalments, including age at diagnosis, body weight, and sex, were not reliable prognostic factors.
Although not helpful in evaluating the prognosis, it is known that small, middle-aged, and female dogs are most commonly affected by MUE [6, 7]. The results of the present study showed a similar distribution.
Several studies of MUE have suggested that seizures are associated with poor prognosis [5, 7, 8, 20]; however, the relationship between seizures and prognosis of MUE was not identified in the present study. This result might be driven by the determination of successful long-term outcomes at a set point of 100 days. Similarly, a study based on 3 months showed the same result as in our study [9]. There was no significant difference in survival analysis according to the presence or absence of seizures, and there was a possibility that anti-seizure treatment was performed well in the present study.
A previous study suggested that sedation further contributes to a decline in brain function [5]; however, there was a possibility that the treatment of severe seizures, such as sedation, was relatively less in the present study.
However, the results of previous studies on CSF analysis as a prognostic factor vary [5, 8]. In contrast to some previous studies that showed that high TP and TNCC in the CSF were associated with an increased risk of death, the present study identified that abnormalities in the CSF analysis was not a reliable prognostic factor [7, 9]. Since the CSF analysis reflects more about the meninges than the parenchyma, it has been reported that sometimes a dog with GME can appear normal. Therefore, there is a possibility that the dogs included in this study had more GME with lesions in the brain parenchyma. Although CSF analysis is known to be more sensitive than MRI in identifying abnormalities consistent with inflammatory disease, normal results of CSF analysis have been described in cases of histopathologically confirmed inflammatory central nervous system disease [2, 4, 20-23].
In the present study, CSF analysis was only performed at the time of initial diagnosis; therefore, it could not be confirmed whether repeated abnormal CSF findings indicate a risk of recurrence [9].
Some previous studies investigating prognostic factors including lesion location and volume, and abnormal MRI features on MRI have reported that when lesions were multifocal or in the brainstem, the prognosis was poor [6]; however, other studies have reported that the location and volume of lesions were not reliable prognostic factors [5, 7, 9, 10]. The results of the present study suggest that the location and volume of abnormal lesions are unreliable prognostic factors.
A previous study reported that a large lesion volume can manifest only as chronic lesions. That is, the larger the lesion, the longer the duration from neurogenic sign onset to MRI diagnosis, and the lesion may indicate non-aggressive disease with slow progression [10].
It was also confirmed that the signal intensity of the lesion varies depending on the degree of inflammation or edema, even in the same lesion volume on MRI, which may be helpful in measuring the intensity of the lesion’s signal on MRI as well as lesion volume. Similarly, the degree of inflammation that affects brain function at that location rather than the location of the lesion may be more important for prognosis, and there is a need to compare the detailed neuroanatomical location with the patient’s clinical signs.
In a previous study, poor prognosis was reported in the presence of effusion of the sulci, cerebellar herniation, and midbrain shift on MRI [9]. However, in another previous dichotomous study on midbrain shift, its presence was not a reliable prognostic factor [8]. The results of this study suggest that although individual abnormal MRI features (such as midline shift, cerebellar herniation, effacement of sulci, and contrast enhancement) were not reliable prognostic factors, the survival time was significantly shorter when more abnormal features were present. Considering the reason why the individual features did not have statistical significance, survival might be affected more by the presence of abnormal MRI features than the respective individual features themselves.
Effacement of sulci, cerebellar herniation, and midbrain shift are indicative of elevated intracranial pressure [9], which may be important for prognosis. For management, especially if there are abnormal MRI features that indicate elevated intracranial pressure, stabilization with decompression therapy (e.g., mannitol, hypertonic saline) and diuretics (e.g., furosemide) might be needed. It might be better to quantitatively evaluate prognostic factors by setting specific rather than dichotomous criteria.
There were statistically significant differences in TNCC in the CSF analysis and lesion volume among the four groups divided based on the treatment drug. As mentioned above, survival was suspected to be unaffected by lesion volume and abnormalities in TNCC in the CSF analysis. In the survival time of the four groups according to the treatment medication, it was suspected that factors other than drug differences did not affect survival. In a previous review article comparing the treatments of MUE [4], the group which was treated with the most diverse drugs had a longer survival time than other groups. The results of the present study also suggest that the addition of various immunosuppressive drugs is effective for the treatment of MUE. This review article reported the use of a combination of PDS and one other drug for continuous treatment without replacement. On the other hand, in this study, drugs were replaced depending on the reaction and side effects.
There was no significant difference in the median survival time between continuous treatment without replacement of drugs [4] and treatment with replacement of various drugs. Early euthanasia was performed and patients with very short treatment periods were included in Groups 1 and 3.
A significant increase in survival time was observed in group 2 (multi-drug) rather than group 1 (cyclosporine alone). Although no significant difference was identified, it was confirmed that the median survival time was long to short in the order of group 2 (multi-drug), group 4 (leflunomide and MMF), group 4 (CA alone), and group 1 (cyclosporine alone). In four dogs in group 1, the lowest therapeutic response was observed. Possible reasons for this result are as follows: 1) cyclosporine acts relatively late compared to other drugs, delaying the effect by 1–2 weeks or more; 2) cyclosporine has difficulty passing through the blood-brain barrier (BBB) and may be less effective if there is less inflammation around the blood vessels and BBB. Moreover, group 1 may have had acute neurogenic disorders, such as disseminated GME or acute NE, with a poor prognosis; 3) some drugs (such as phenobarbital) may have reduced the effectiveness of cyclosporine. These findings suggested that CA-based multi-drug therapy in dogs with MUE could be considered as first-line treatment because CA was more effective than cyclosporin, and the highest median survival time was in group 2, where multiple immunosuppressants were administered.
In conclusion, abnormal MRI features were associated with poor prognosis in dogs with MUE. In addition, CA-based multi-drug therapy might be effective in dogs with MUE, and replacement with immunosuppressive drugs might be considered in MUE cases with drug resistance and adverse effects.
This study has some limitations owing to its retrospective design. First, the inclusion criteria were based on previously reported studies and the sample size was relatively small in the present study. Because of the lack of histopathology, the MUE subclass was not analyzed. It was difficult to accurately investigate the prognostic factors in dogs with MUE because the immunosuppressive drugs used in the treatment were different. Medical management was also tailored to individual needs; therefore, some dogs might have received additional medications such as anticonvulsant drugs, mannitol, furosemide, and antibiotics. In addition, MRI findings, results from the CSF analysis, and drug concentration were not analyzed repeatedly, and disease response and recurrence were not evaluated. If these are repeatedly analyzed and compared with objective indicators such as neurological examination and survival, it would be more helpful to evaluate the prognosis and clinical outcome in MUE.







