Introduction
Mitral valvular prolapse (MVP) in dogs is characterized by myxomatous valvular degeneration, which is caused by abnormal valvular thickening and incomplete coaptation of the mitral valve leading to mitral regurgitation [3-5, 10]. MVP constitutes up to 75% of all canine heart diseases [6]. Clinical signs and prognosis can differ depending on risk factors such as severity of valve lesions, age (older), and gender (male than female) [10, 17]. Although the pathogenesis of MVP is unknown, some studies have suggested the involvement of endothelin and systemic connective tissue diseases [10]. MVP in dogs commonly occurs in aged small dog breeds, including Chihuahuas, Malteses, Shih Tzus, Yorkshire terriers, Dachshunds, and Cavalier King Charles Spaniels (CKCS) [3, 10]. MVP in dogs shows high breed predisposition and genetic predisposition [9, 10, 12-14, 17]. Specifically, MVP is about 20 times more prevalent in CKCS than other dog breeds [3]. Familial MVP has been observed in Dachshunds and CKCS [9, 12-14], although the exact gene mutation and inheritance pattern have not been clearly identified. If dogs show severe clinical signs of MVP, therapy for heart failure is required even though the prognosis is grave due to refractory heart failure [2]. In dogs with MVP, the mitral valve acts as a one-way valve that allows blood to flow into the ventricles during ventricular diastole while preventing blood from flowing backward into the atria during ventricular systole. Mitral valvular insufficiency can lead to blood regurgitation into the left atrium (LA), causing secondary morphologic and hemodynamic changes. Major clinical signs associated with heart failure due to MVP are as follows: (1) respiratory distress and coughing from pulmonary edema and main-stem bronchial compression due to increased left atrial and pulmonary venous pressure; (2) weakness and lethargy from reduced left ventricular or right ventricular forward flow; (3) pleural effusion and ascites from right-sided heart failure; (4) acute decompensation with fulminant pulmonary edema or ventricular fibrillation, which may cause sudden death; (5) syncope (possibly related to tachyarrhythmia or vasovagal syncope) [10].
In humans, MVP is also a common cardiac abnormality that occurs either as an isolated anomaly or as part of a generalized connective tissue disorder, especially Marfan syndrome [1]. Isolated MVP in humans is mostly sporadic, although autosomal dominant or X-linked forms have been described [8, 9, 11]. A recent study in humans found that the gene encoding filamin A (FLNA) located on the X-chromosome is responsible for familial MVP [11]. Other studies have identified loci on chromosomes 11, 13, and 16 linked to familial MVP [8, 9].
In this study, we identified for the first time putative familial MVP in a Maltese dog family. All family members were in different stages of heart failure due to mitral valve prolapse. This case study investigated the clinical features of an affected Maltese family and performed pedigree analysis.
Case report
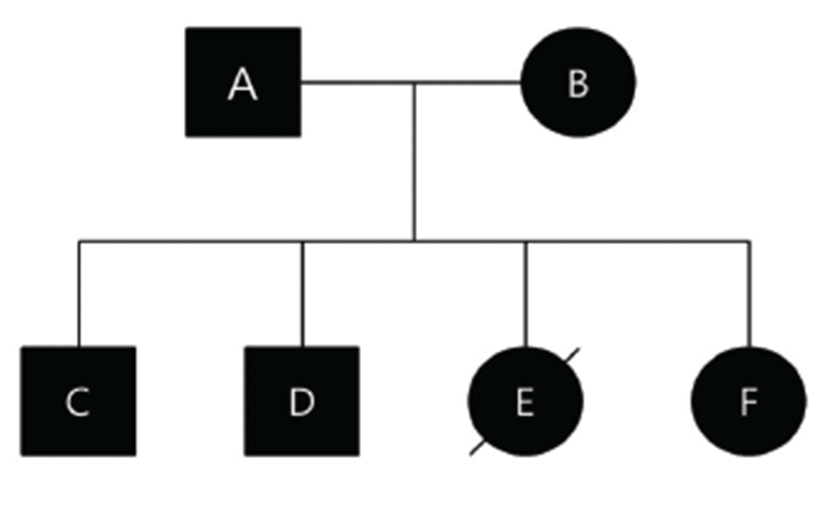
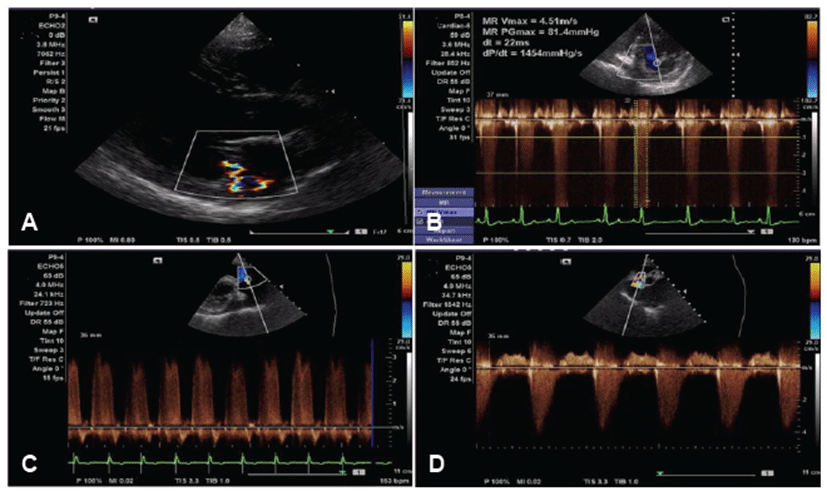
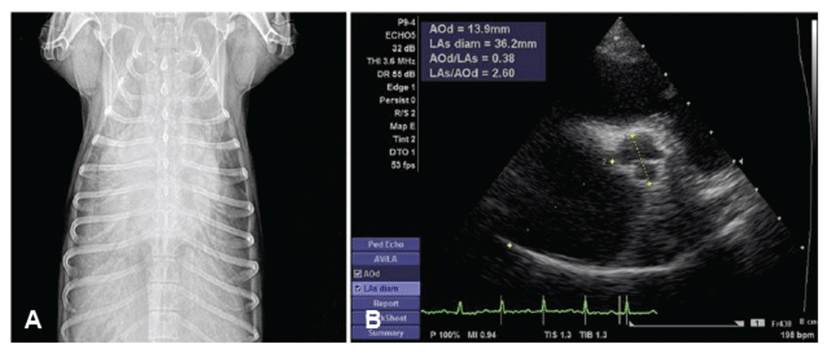
Prior to this study, we obtained approval of the Animal Ethics Committee of Kangwon National University for cardiac examination and pedigree analysis in this family of dogs. Informed written consent, including information pertinent to our investigation, was obtained from dog owners prior to commencement of the study. Affected parents and their offsprings were all in different households. The affected Maltese family consisted of a father (A), mother (B), two sons (C, D), and two daughters (E, F) (Fig. 1). Dog A had been diagnosed with mitral regurgitation (MR) at 9-years-old, and the clinical stage was ISACHC Ib at that time. The progression to heart failure was slow, and clinically obvious heart failure (ISACHC II) was detected from the age of 11. Tricuspid regurgitation (TR) and pulmonary hypertension (PHT) were detected from an age of 13 years (Fig. 2). Supraventricular tachycardia with sinus arrest was also detected from the age of 13. Current clinical status of this dog (age of 14) is ISACHC IIIa. Dog B had been diagnosed with mitral regurgitation (MR) at an age of 10, and the clinical stage was ISACHC Ib at that time. Disease progression of dog B was faster than that of dog A. Clinically obvious heart failure (ISACHC II) was detected from the age of 11. TR and cardiac conduction disturbances have not yet been detected in this dog. Current clinical status of this dog (age of 12) is ISACHC IIIa. All offsprings (dogs C, D, E, and F) showed heart murmurs from an age of 6. Clinically obvious heart failure due to MVP was detected between 7 to 8 years of age in these dogs. Dog E died of fulminant pulmonary edema from congestive heart failure at the age of 10 (Fig. 3). The rest of the dogs all survived. Current clinical status of dogs (sons) C and D is ISACHC IIIa, whereas that of dog (daughter) F is ISACHC IIIb. Dog F had been diagnosed with hypoadrenocorticism at the age of 9 and was routinely administered desoxycorticosterone acetate (DOCP). All affected dogs are currently being medicated with different dosages of furosemide, pimodendan, enalapril, and spironolactone. Dog A also currently receives sildenafil for controlling PHT. Clinical and diagnostic features for this affected family are summarized in Table 1.
Discussion
To the best of the authors’ knowledge, this is the first report of putative familial mitral valve prolapse and regurgitation in Maltese dogs. All family members in this study showed degenerative valvular changes and echocardiographic features of MVP. Although disease progression differed, all dogs progressed to advanced heart failure stage within 2~3 years after diagnosis. One recent retrospective study investigated the clinical features of MVP in 207 Dachshunds [6]. Of these, 172 dogs had only MR, 39 dogs had MR and TR, and three dogs had only TR. The study also found that males were more frequently affected, and the average age of dogs with MVP was around 11 to 12-years-old. A majority of the affected Dachshunds were classified as ISACHC I and II. Lastly, the study found that MVP in Dachshunds was associated with late onset of clinical signs and few cardiac complications [6]. The clinical features and disease progression in our affected Maltese family was quite similar to those in Dachshunds with MVP.
MVP in Cavalier King Charles Spaniels (CKCS) is also common. One survey measured a mortality rate of 42.8% due to cardiac causes in United Kingdom CKCS [12]. In CKCS, there are two types of MVP: an ‘early onset’ group with a detectable murmur before 5 years of age and a ‘late onset’ group with no detectable murmur up to 7 years of age. However, CKCS tends to show a severe early onset form of MVP [3, 12]. One recent study revealed that CKCS with early onset MVP have genetic etiologies with 0.3 and 0.6 heritability of heart murmurs [12]. Unfortunately, the exact genetic cause has not been found in dogs with MVP, although one recent study was able to identify genetic factors in the etiology of MVP in CKCS using genome-wide screening technologies [14]. Several genetic etiologies for inherited MVP have already been found in humans [7, 8, 11].
It is unclear whether the genetic etiology of Maltese dogs with MVP is the same as that of CKCS with MVP. Clinical features of familial MVP in the Maltese parent were similar to those in aged dogs with degenerative mitral valvular disease. However, clinical features of the Maltese offspring in this study were more similar to the early onset form of MVP in CKCS. Since clinical features differed between the parent and their offsprings, the parent might be heterozygous for the responsible gene mutation, and thus experience slowed disease progression, whereas the offsprings might be homozygous and thus experience hastened disease progression. Maine Coon and Ragdoll cats show similar clinical features of familial hypertrophic cardiomyopathy [15, 16]. Heterozygous cats for MYPBC3 gene mutation show slower and milder clinical signs than homozygous cats. However, this explanation might be against the laws of Mendelian inheritance, as there was no unaffected Maltese offspring in this family. Environmental factors might also be involved in the progression of MVP in our family. It is unfortunate that all members of this dog family were affected by MVP, which means we could not evaluate the actual inheritance pattern. It is possible that environmental factors, including diet, water supply, and salt content, caused development of MVP in this dog family. However, all affected dogs were from different households, and thus this chance is quite low. However, the different clinical features among the family members might be due to different breeding environments. Unfortunately, there is no way to confirm the responsible gene mutation in this family, as the actual genetic etiology has not yet been defined in dogs, including CKCS and Dachshunds. Further study should be conducted to unveil the actual genetic etiology and inheritance pattern in Maltese dogs with MVP.
In conclusion, we have for the first time identified putative familial mitral valve prolapse in Maltese dogs. This finding suggests strong genetic etiology involved in the development of degenerative mitral valve disease in dogs. Furthermore, this finding could be a valuable resource for the identification of gene mutations in dogs with familial MVP.







